Antibodies recognizing tau
An antibody, humanized antibody technology, applied in the direction of antibody medical components, medical preparations containing active ingredients, anti-animal/human immunoglobulin, etc., can solve problems such as interference with microtubule assembly, neuronal network damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0571] Example 1. Identification of tau monoclonal antibody
[0572]Monoclonal antibodies against tau were generated as follows. Recombinant 383a.a. human tau (4RON) with an N-terminal His tag containing the P301S mutation [Immunogen A] or recombinant 383a.a. human tau (4RON) containing the P301S mutation lacking the N-terminal His tag [Immunogen B] Perform immunization. The immunogen was emulsified in RIBI adjuvant.
[0573] Five-week-old female Balb / c mice were immunized intraperitoneally with 25 μg of immunogen A on day 0 and with 10 μg each of immunogen on days 7, 14, 21, 27, 34, 48, 55, and 62 A Intraperitoneal immunization was performed. Mice were immunized with 10 μg of immunogen B on days 76 and 90. On days 43 and 98, mice were bled and titrated against immunogen A; on day 101, the animal with the highest titer was boosted with a terminal immunization of 50 μg of immunogen B, the Immunogen B was delivered 1 / 2 ip and 1 / 2 iv. Fused hybridomas were screened against ...
Embodiment 2
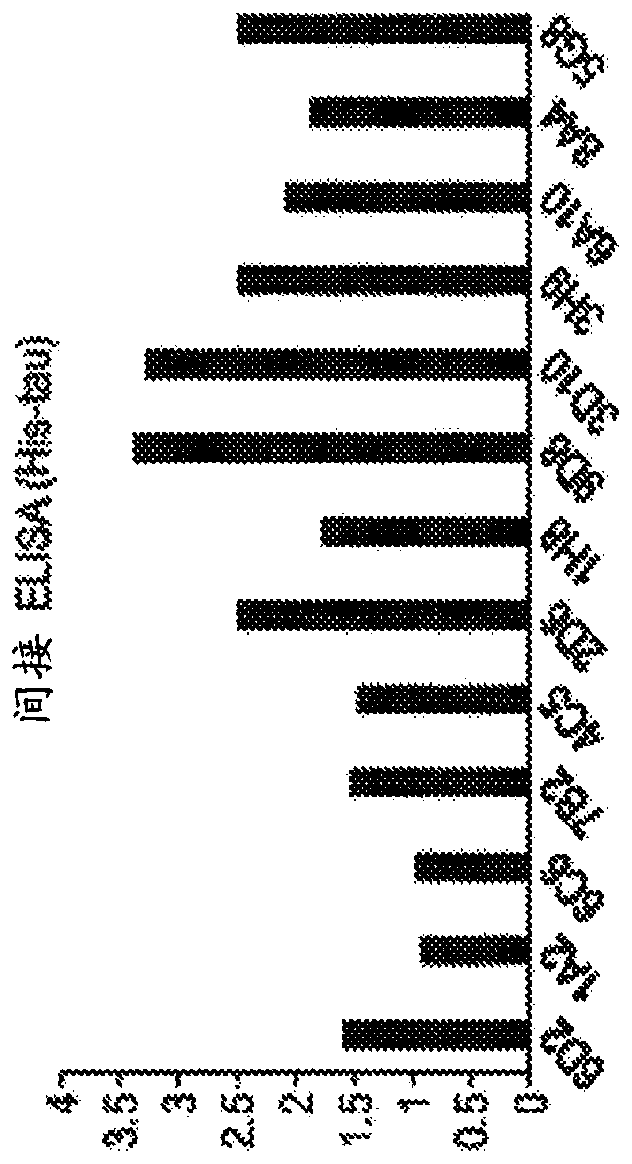
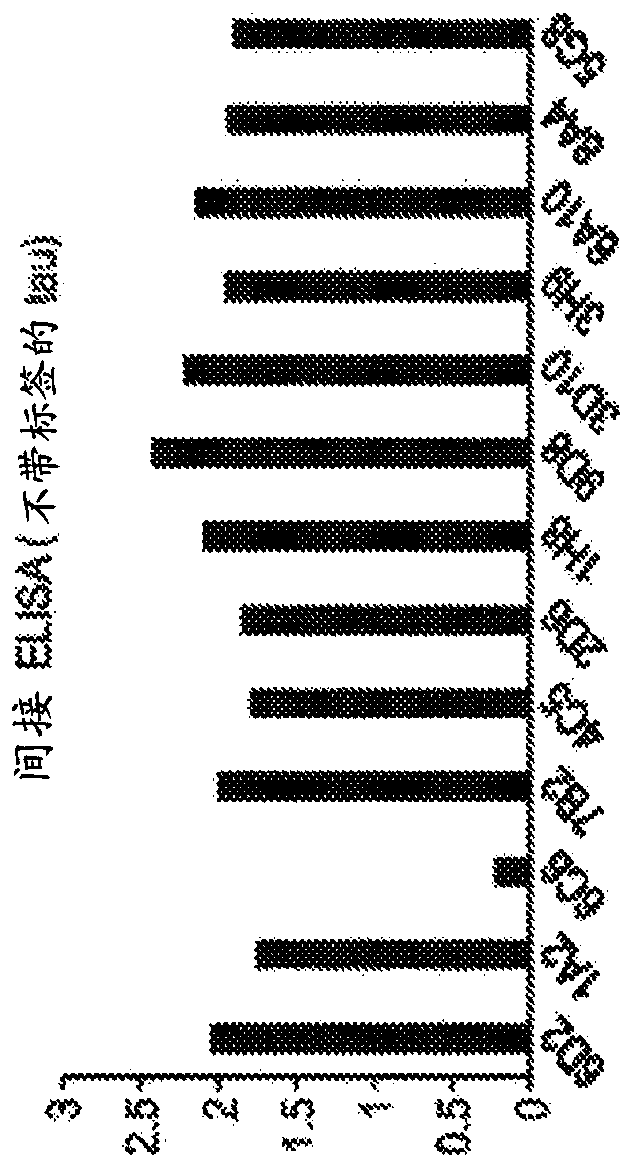
[0574] Example 2. Binding of mouse monoclonal antibodies to tau in an ELISA assay
[0575] Method: Indirect ELISA: 96-well polystyrene plate with capture antibody anti-6xHis ( Figure 1A ) or polyclonal anti-tau (Dako#A0024, Figure 1B ) coating for 2h at room temperature or 16h at 4°C. The coating was removed, the plate was blocked with 1% BSA in 1xPBS for 1 h, and then incubated with human recombinant tau, which has a polyhistidine tag at the N-terminus of the protein ( Figure 1A ) or without ( Figure 1B ) polyhistidine tag. After washing, plates were incubated with the indicated antibodies, washed, and incubated with HRP-conjugated goat anti-mouse secondary antibodies. Plates were developed with TMB and A was measured with a plate reader 450 .
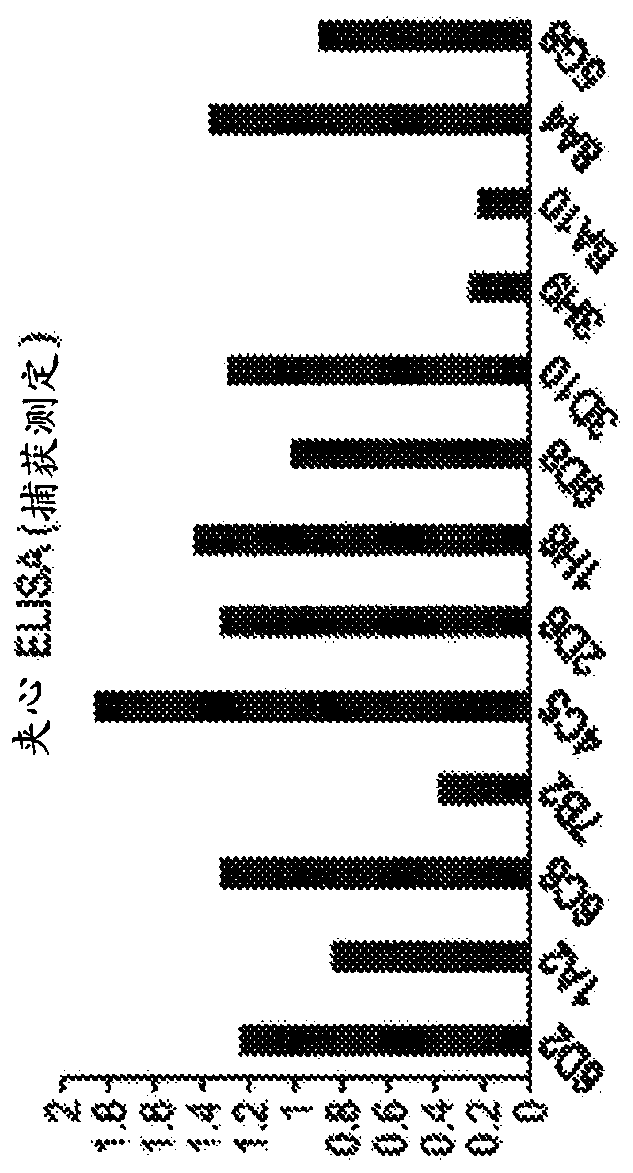
[0576] Sandwich ELISA: 96-well polystyrene plates were coated with anti-mouse antibody in 1xPBS for 2h at room temperature or 16h at 4°C. The coating was removed and the plate was blocked with 1% BSA in 1xPBS for 1 h. Plates...
Embodiment 3
[0578] Example 3. Affinity of mouse monoclonal antibodies to tau
[0579] Methods: SPR analysis was performed using a Biacore T200 to determine the binding kinetics of murine antibodies to recombinant human tau. To prepare the sensor surface, anti-mouse antibody (GE Life Sciences) was immobilized on the sensor chip CM5 by amine coupling, and the antibody was captured at a level to ensure maximal binding of 50RU. Various concentrations of recombinant tau ranging from 10-0.14 nM in running buffer (HBS + 0.05% P-20, 1 mg / mL BSA) were passed over the capture ligand at a flow rate of 50 μL / min for 180 sec association and 900sec dissociation. Data were double referenced to an irrelevant sensor without antibody ligand and OnM analyte concentration to account for ligand dissociation from the capture moiety. Data were then analyzed using a global 1:1 fit.
[0580] Results: Multiple murine antibodies were selected based on their performance in a battery of ELISA assays and their bind...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap