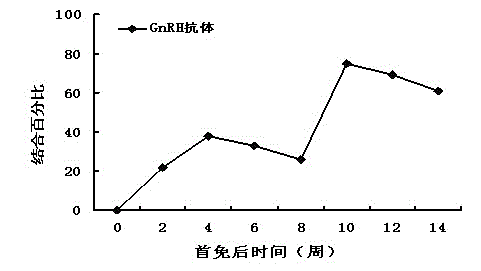
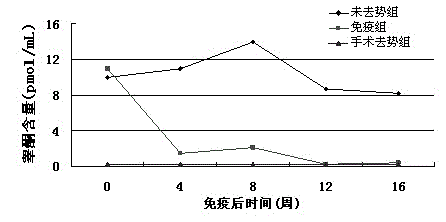
Recombinant phage peculiar smell removal vaccine for boars
A phage and odor-removing technology, applied in the direction of virus/phage, recombinant DNA technology, drug combination, etc., can solve problems affecting pig growth performance, etc., and achieve the effect of being convenient for large-scale production, easy to cultivate, and satisfying feeding management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Construction of recombinant phage
[0042] A DNA sequence was synthesized by a commercial company. The DNA sequence has been cloned into the multiple cloning site of the pUC-19 vector. The 5' end of the DNA sequence has a restriction site EcoRI, and the 3' end contains a restriction site HindⅢ. The DNA sequence As shown in SEQ ID NO: 3
[0043] The above DNA sequence was excised from the pUC-19 vector with restriction endonucleases EcoRI and HindIII and inserted between EcoRI and HindIII of the T7 phage multiple cloning site to construct a recombinant phage.
[0044] Recombinant pUC-19 vector double enzyme digestion system:
[0045] wxya 2 o 16.0 μL 10×H Buffer 5.0 μL pUC-19 vector 25.0 μL EcoRI 2.0 μL HindⅢ 2.0 μL
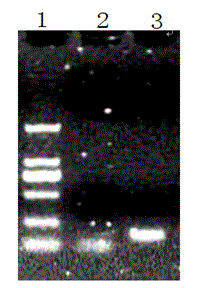
[0046] Mix the above reaction system and place it in a 37 °C water bath for 4 h. After the digested products are also identified by 1% agarose gel electrophoresis, the gel recovery kit is used for gel...
Embodiment 2
[0058] Example 2 Large scale amplification of recombinant phage
[0059] Glycerol frozen E. coli Strain BL21 was inoculated on the plane of LB medium, cultured overnight at 37 °C; a single colony was picked from the plate, inoculated with 5 mL of LB culture medium, and cultured overnight at 37 °C with shaking at 200 rpm. Take 3mL overnight culture to inoculate 300mL LB culture medium, cultivate to OD 600 =0.8 or so. Pick a single phage plaque on a plate, inoculate it into the cultured BL21 host bacteria, and incubate with shaking at 37°C and 100 rpm for 2-3 hours until the bacterial solution changes from turbid to clear. The phage was recovered by PEG-NaCl precipitation, and the phage titer was determined according to the conventional method. Adjust the recovered phage concentration to 2×10 13 pfu / mL, and add formaldehyde solution at a ratio of 4‰, and inactivate overnight at 37°C and 100 rpm with shaking.
Embodiment 3
[0060] Example 3 Preparation of phage deodorant oil emulsion vaccine
[0061] The preparation method of recombinant phage deodorizing oil emulsion vaccine is as follows:
[0062] Preparation of the oil phase of the vaccine: 4mL Siben 80, 2mL Siben 85, 94mL No. 10 mineral oil, 2g aluminum stearate, mix well, and press at 121°C for 20 minutes under high pressure. Preparation of vaccine aqueous phase: 94mL titer is 2×10 13 Pfu / mL of inactivated phage, 4 mL of autoclaved Tween-80, 3000 rpm and stir well. According to the ratio of water phase: oil ratio of 1:3, add 150mL oil phase to the tissue masher, slowly add 50mL water phase while stirring slowly, after mixing well, stir at 10000 rpm / quickly for 2 minutes until a stable The water-in-oil structure is the prepared recombinant phage oil emulsion vaccine, which is stored at 4°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com