Antibodies recognizing tau
A technology of antibody and humanized antibody, which is applied in the direction of antibodies, medical preparations containing active ingredients, anti-animal/human immunoglobulin, etc., and can solve problems such as interference with microtubule assembly and neuronal network destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0516] Example 1. Identification of TAU monoclonal antibody
[0517] A monoclonal antibody for TAU is produced as follows. The 383A.A.Aman Tau (4R0N) [immunogen a] or P301S mutation is lacking the N-terminal His label, lack of N-terminal His label, lack of N-terminal His label, 383A.A. People TAU (4R0N) [Immunogen B] Perform immunization. Immunogen was emulsified in Ribi adjuvants.
Embodiment 2
[0519] Example 2. Top of the antibody 3D6
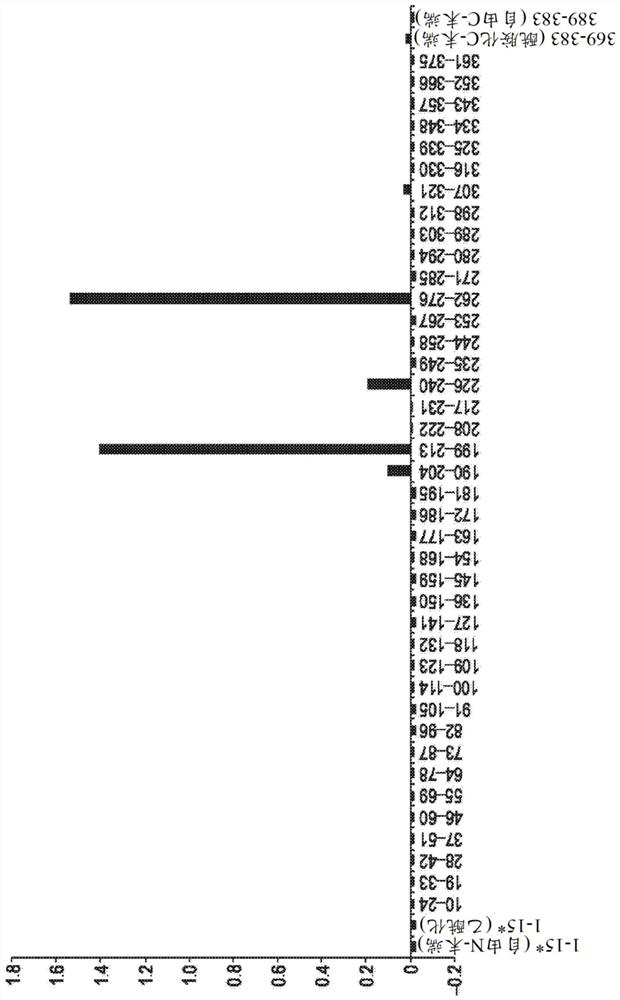
[0520] A series of overlapping biotinidin peptides across the entire 383AA 4R0n Tau protein were used to make a figure 3D6 antibody. Additional peptides are used to model the potential translational modification of the C-terminus of the protein.
[0521] Biotininic peptide binds to a separate hole of the ELISA tablet of the streptavidin package. The plate was closed and treated with murine 3D6, and then incubated with a mouse antibody conjugated to a horseradish peroxidase. After washed thoroughly, OPD is applied to the tablet and developed. Read the absorbance at 450 nm at 450 nm. The background is reduced by the absorbance value without one-resistant hole, and the threshold of the positive binding is set to 0.2 absorbance units.
[0522] The peptide binding is detected on peptides across (SEQ ID NO: 3) amino acid residues 199-213 and (SEQ ID NO: 3) amino acid residue 262-276. The number of the total length of 4R2N Tau protein (441 amin...
Embodiment 3
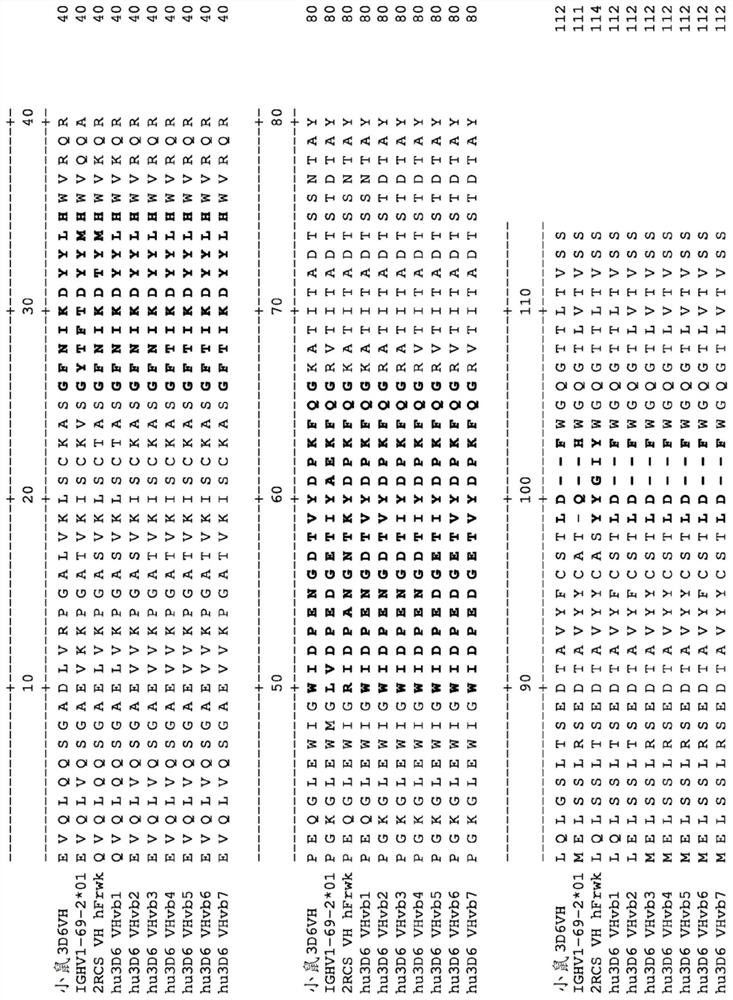
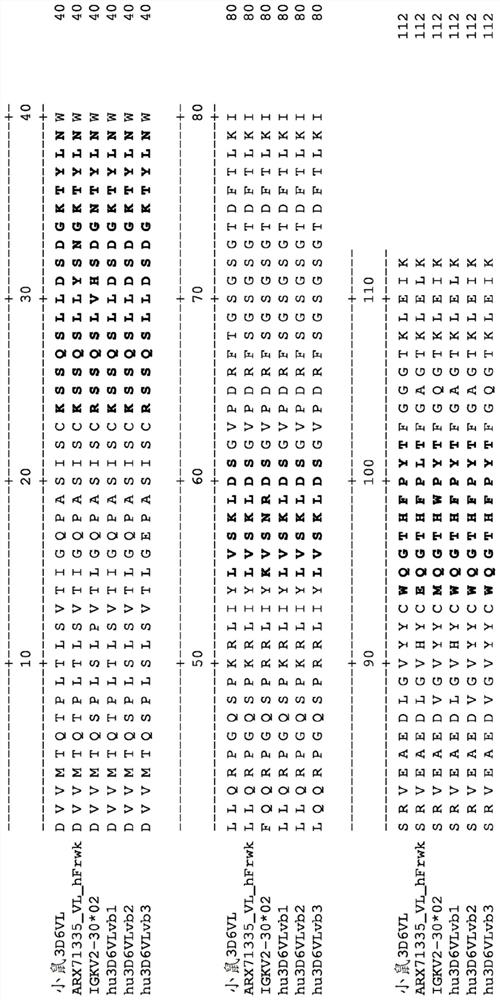
[0523] Example 3. Design of humanized 3D6 antibody
[0524] The humanized starting or donor antibody is mouse antibody 3D6. Mature M3D6's heavy chain variable amino acid sequence is provided as SEQ ID NO: 7. Mature M3D6 light chain variable amino acid sequence is provided as SEQ ID NO: 11. The heavy chain Kabat / Chothia complex CDR1, CDR2 and CDR3 amino acid sequences are provided as SEQ ID NO: 8-10, respectively. The light chain Kabatcdr1, CDR2 and CDR3 amino acid sequences are provided as SEQ ID NO: 12-14, respectively. Always use the Kabat number.
[0525] 3D6 variable κ (VK) belongs to the Kabat 2 subgroup of mice, which corresponds to the Kabat 2 subgroup, and the variable heavy chain (VH) belongs to the kabat 2C subgroup of mice, which corresponds to human kabat 1 subgroup [Kabat EA et al, (1991), Sequences of Proteins of Immunological Interest, Fifth Edition NIH Publication 91-3242]. In VK, 16 residue Chothia CDR-L1 belongs to the specification category 4,7 residue Chothia...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap