IGFBP3 antibodies and therapeutic uses thereof
An antibody and antigen technology, applied in the field of IGFBP3 antibody and its therapeutic application, can solve problems such as not being ideal for antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0290] Example 1: Monoclonal Antibody Development
[0291] Monoclonal anti-IGFBP3 antibodies were discovered using transgenic mice in which the relevant human immunoglobulin sequences have been genetically engineered (Trianni Mouse TM (Trianni)) were introduced into animal genomes.
[0292] Through this technique, chimeric monoclonal antibodies are produced that contain a complete repertoire of human heavy and light chain variable domains and retain mouse constant domains.
[0293] Basically, the Trianni Mouse of the two cohorts TM (Cohort 1: ALD / MDP adjuvant, Cohort 2: SAS / Ribi adjuvant) Immunization with a purified preparation of IGFPP3 antigen (Lot AB08BP1210) was injected twice weekly for 4 weeks, then extended by 2 weeks. Then, antibody-expressing lymphocytes (such as B cells) are recovered from the mice, such cells are fused with myeloid cell lines to prepare immortalized hybridoma cell lines, and such hybridoma cell lines are screened and selected, Hybridoma cell lin...
Embodiment 2
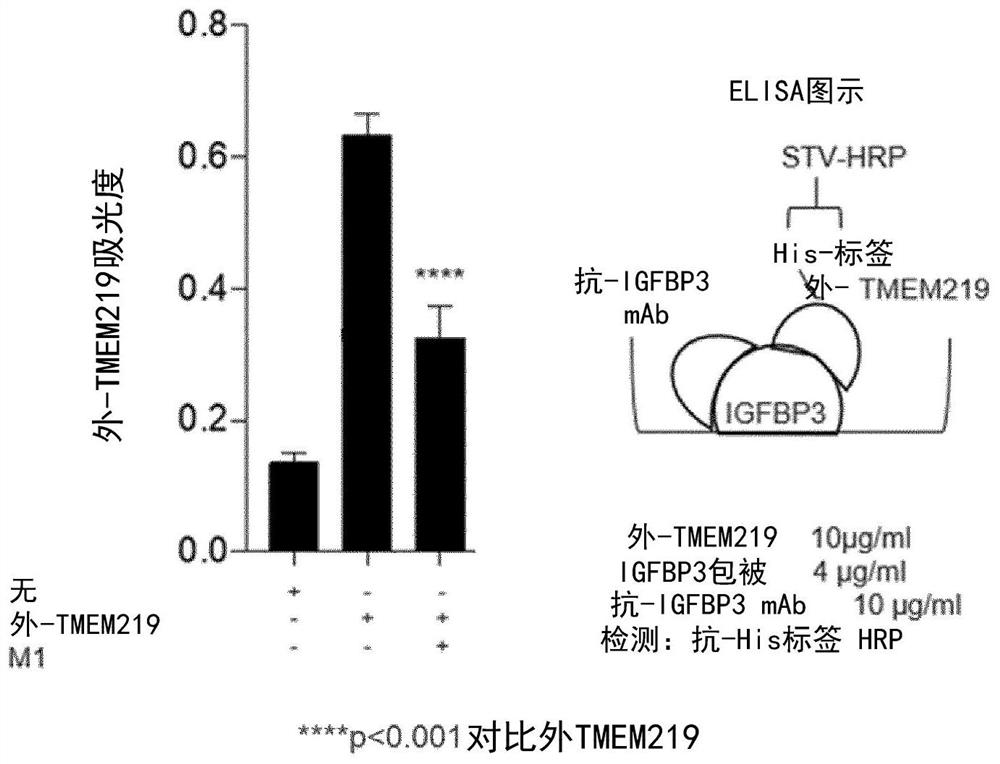
[0336] Inhibition of IGFBP3-TMEM219 binding by hybridoma-produced anti-IGFBP3 mAb
[0337] Novel anti-IGFBP3 monoclonal antibodies produced using hybridomas were screened for their ability to compete with exo-TMEM219 for interaction with IGFBP3 using a competitive ELISA binding assay. IGFBP3, exo-TMEM219 and available antibodies were all used in a 1:1 ratio. Anti-IGFBP3 mAb was able to inhibit IGFBP3-exo-TMEM219 ( figure 1 ). This demonstrates that the anti-IGFBP3 mAbs of the present invention can inhibit the binding of IGFBP3 to the native TMEM219 receptor and may mimic the neutralizing activity of exo-TMEM219 protein.
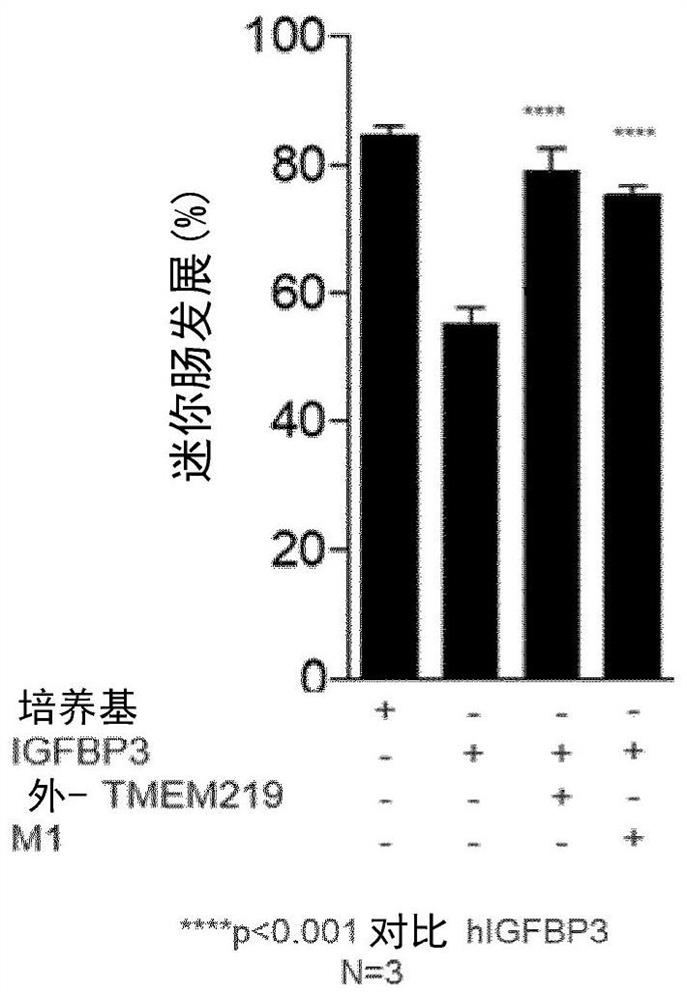
[0338] A newly generated monoclonal anti-IGFBP3 antibody rescues IGFBP3-damage in a human mini-gut assay
[0339] The newly discovered monoclonal antibody was also tested in a mini-gut test. Briefly, mini-guts were generated from crypts obtained from healthy controls (n=3), cultured in the presence of IGFBP3 for 8 days and treated with exo-TMEM219 or ...
Embodiment 3
[0356] A newly generated monoclonal anti-IGFBP3 antibody rescues IGFBP3-damage in a human mini-gut assay
[0357] Anti-IGFBP3 monoclonal antibodies were tested in the mini-gut assay. Briefly, mini-guts were generated from crypts obtained from healthy controls (n=3), cultured for 8 days after IGFBP3 exposure, and treated with anti-IGFBP3 mAb at a 1:1 ratio (mAb:IGFBP3). The inventors observed that E08 and E20 were comparable to exo-TMEM219 in the self-renewal ability to rescue large crypt organoids in the presence of IGFBP3 among the 8 mAbs, thus supporting protection against IGFBP3-mediated damage on local stem cells. Correlation effect ( Figure 8 ).
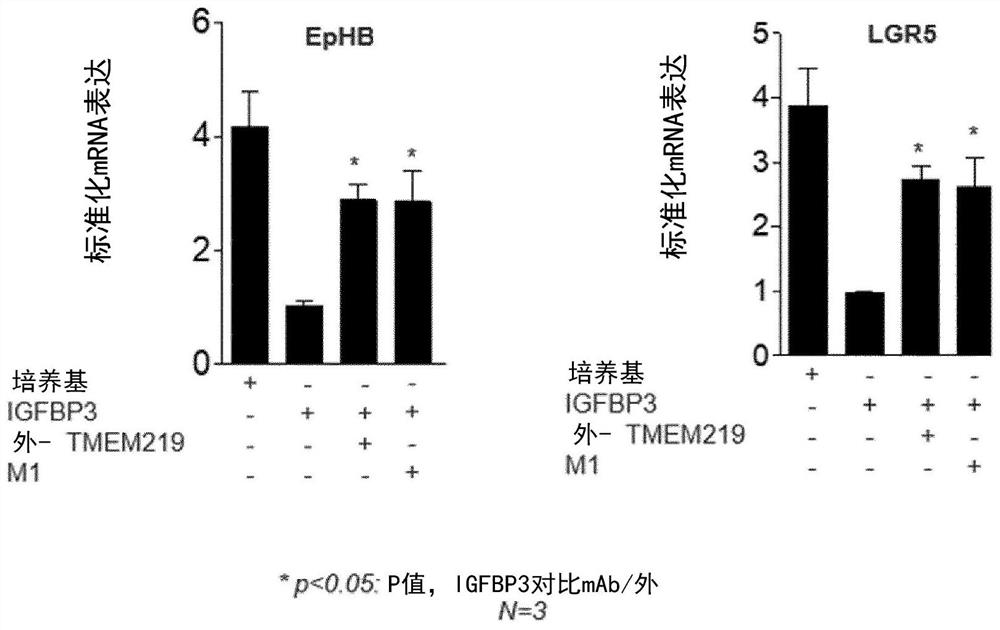
[0358] A newly generated monoclonal anti-IGFBP3 antibody rescues IGFBP3-damage on ISC markers
[0359] Anti-IGFBP3 mAb, which was shown to effectively promote mini-gut development, was also able to restore the expression of ISC markers EphB2 and LGR5 ( Figure 9 ). This effect was caspase 8-mediated, as caspase 8 expre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com