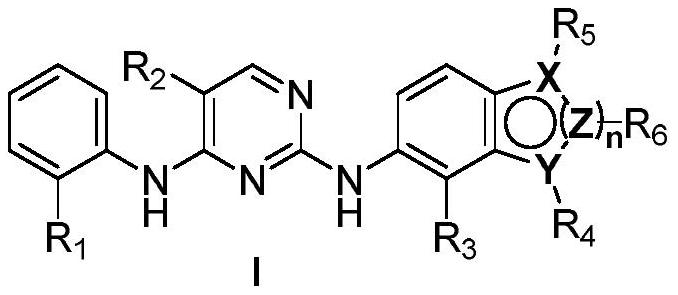
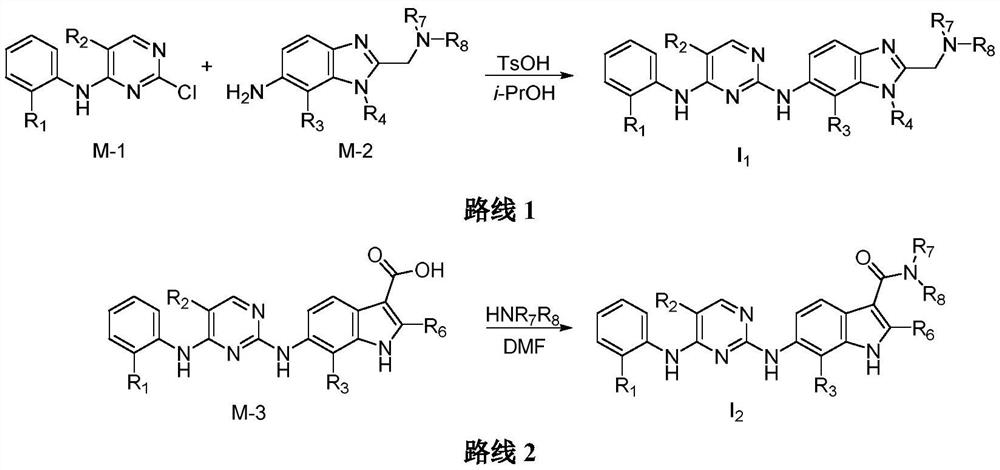
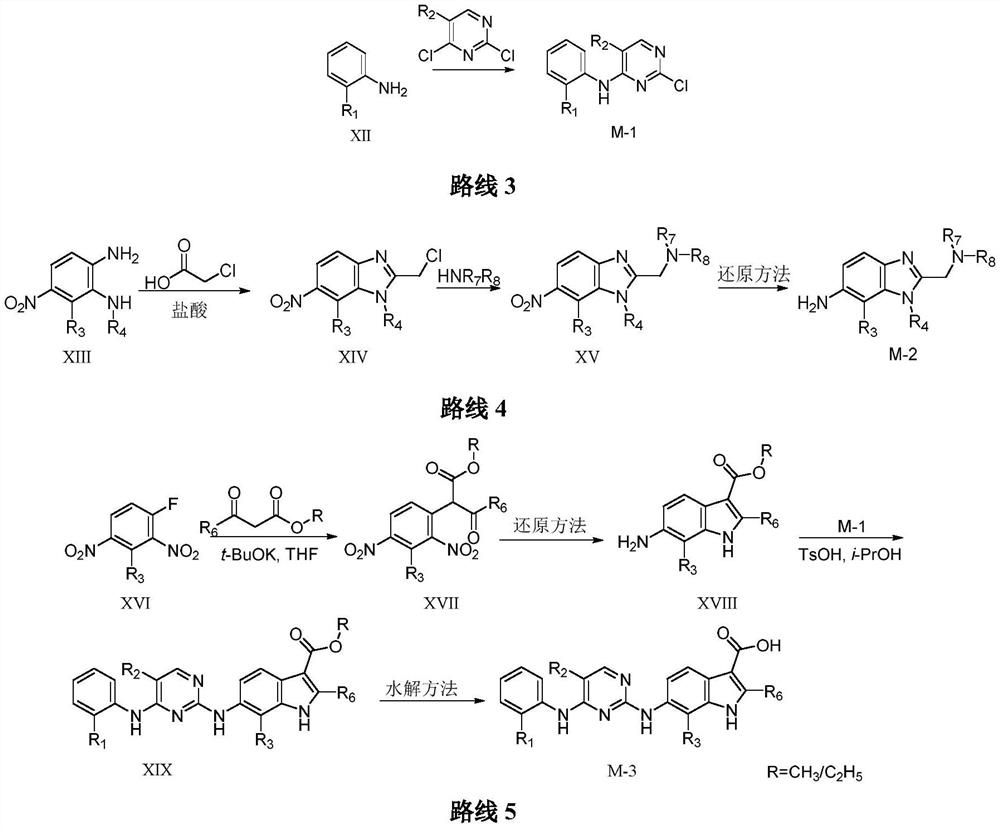
2,4-diarylaminopyrimidine derivatives containing aromatic heterocycles and their preparation and application
A technology of arylaminopyrimidine and aromatic heterocycle, applied in the field of medicine, can solve problems such as ROS1 protein imbalance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] Step A N- (2,5-dichloronidin-4) -1,2-diphenylamine (III)
[0241] 30.00 g (0.16 mol) orthodontisylamine (II) and 17.70 g (0.16 mol) 2,4,5-triclactane were added to 300 ml of dried isopropanol (I-prOH), and 42.30 g slowly added ( 0.33 mol) N, N-diisopropylethylamine, warmed to 80 ° C for 1.5 h. The filtration was filtered, and the white powder solid was 37.35 g, and the yield was 89.7%.
[0242] Step B N- (2 - (((2,5-dichloroprid-4-yl) amino) phenyl) methanesulfonamide (M 1 -1)
[0243] Under ice bath conditions, 10 g (0.04 mol) intermediate III and 9.3 g (0.12 mol) pyridine (PY) were added to 50 ml of dried tetrahydrofuran, slowly added 5.4 g (0.05 mol) methylsulfonyl chloride. Tipping, at room temperature for 2.5 hours, filtration, tetrahydrofuran (10 mL × 3) washed, and the white powder solid was 11.7 g, and the yield was 89.3%.
[0244] Step C 2- (chloromethyl) -6-nitro-1H-benzo [D] imidazole (VI)
[0245] 10.0 g (0.07 mol) of 4-nitro-xylomide (V), 12.3 g (0.13 mol) chlo...
Embodiment 2
[0253] Example 2N- (2 - ((5- chloro-2 - ((2 - ((4- (2-hydroxyethyl) piperazin-1-yl) methyl) lH-benzo [d] imidazole - 6-yl) amino) pyrimidine-4-yl) amino) phenyl) methanesulfonamide
[0254] According to the method of step d in Example 1, the N-hydrocarbonization reaction was prepared from N-hydroxyethylpiperazine by VI to obtain 2- (4 - (((6-nitro-1H-benzo [D] Imidazole-2-yl) methyl) piperazine-1-yl) ethyl-1-alcohol (VII-2). The yield was 83.0%.
[0255] According to the method of step e in Example 1, it is obtained by the reduction reaction to obtain 2- ((6-amino-1H-benzo [D] imidazole-2-yl) methyl) piperazine by the reduction reaction at VII-2. -1-yl) ethyl-1-alcohol (M 2 -2), the yield is 97.8%.
[0256] In accordance with the method of step f in Example 1, 1 -1 and M 2 -2 is raw material, prepared Example 2, and yields 80.5%.
[0257] M.p.:237.6-240.2 CAE (es) + (m / z): 572.46; 1 H NMR (400MHz, DMSO-D 6Δ12.05 (S, 1H), 9.27 (S, 1H), 8.63 (S, 1H), 8.18 (D, J = 7.8 Hz, 1H), 8.15...
Embodiment 3
[0258] Example 3 N- (2 - ((5-chloro-2 - ((2 - (dimethylamino) methyl) -1H-benzo [D] imidazole-6-yl) amino) pyrimidine-4- Base) amino) phenyl) methanesulfonamide
[0259] According to the method of step d in Example 1, N-hydrocarbonization reaction was prepared from dimethylamine (40% aqueous solution) at VI, N-hydrocarbonization reaction was prepared from N-hydrocarbonization reaction to N, N-dimethyl-1- (6-nitro-1H- Benzo [D] imidazole-2-yl) methylamine (VII-3). The yield was 96.2%.
[0260] According to the method of step e in Example 1, the reacting reaction was obtained by the reduction reaction by the reduction reaction to obtain 2 - ((dimethylamino) methyl) -1H-benzo [D] imidazole-6-amine (M) by the reduction reaction. 3 -2), the yield is 92.6%.
[0261] In accordance with the method of step f in Example 1, 1 -1 and M 3 -2 is raw material, preparation Example 3, yield 95.9%.
[0262] m.p.:212.3-215.1 CA es es -[[m +h] + (m / z): 487.28; 1 H NMR (400MHz, DMSO-D 6 Δ12.14 (S, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com