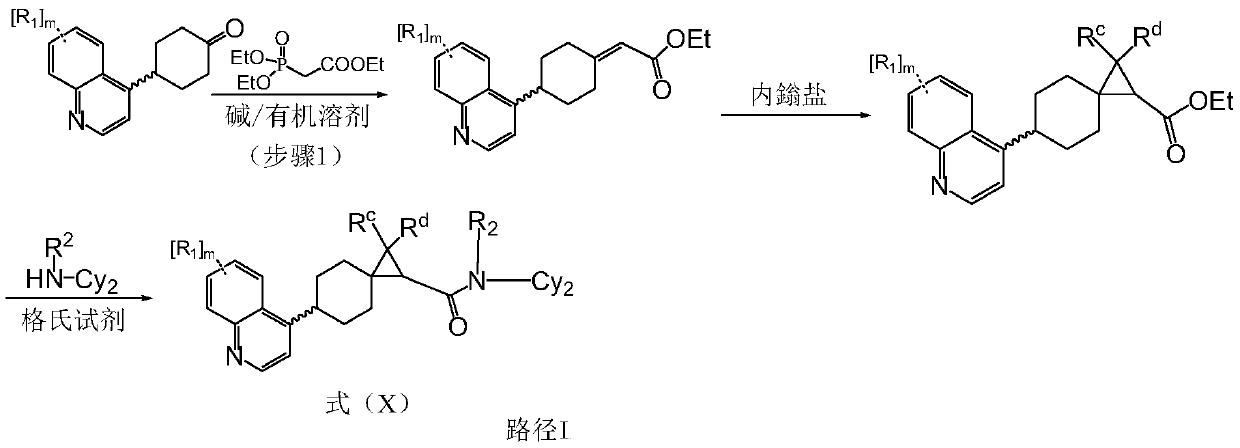
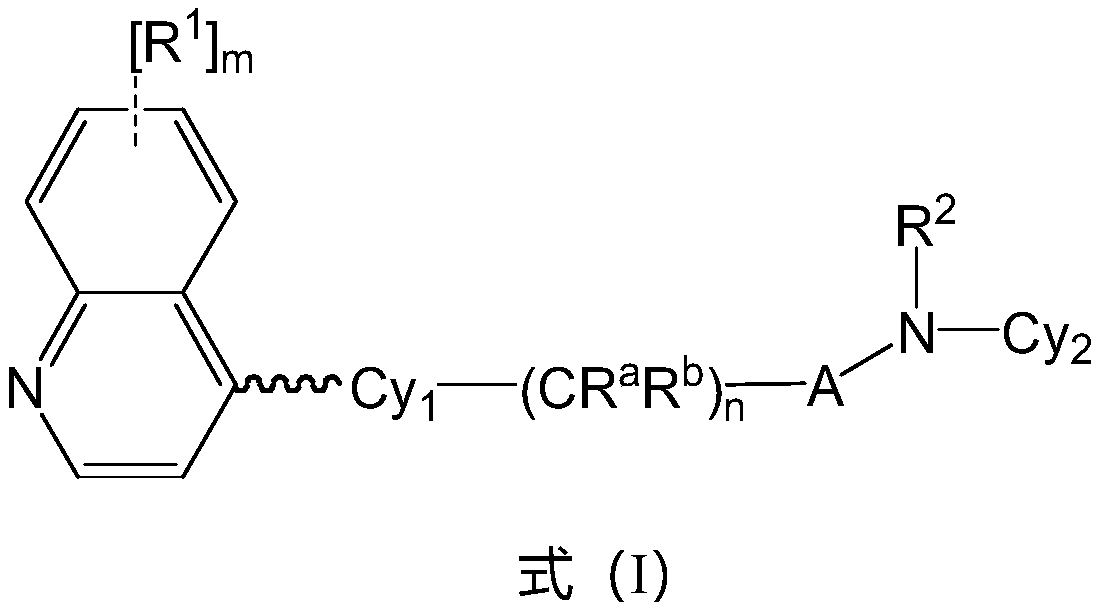
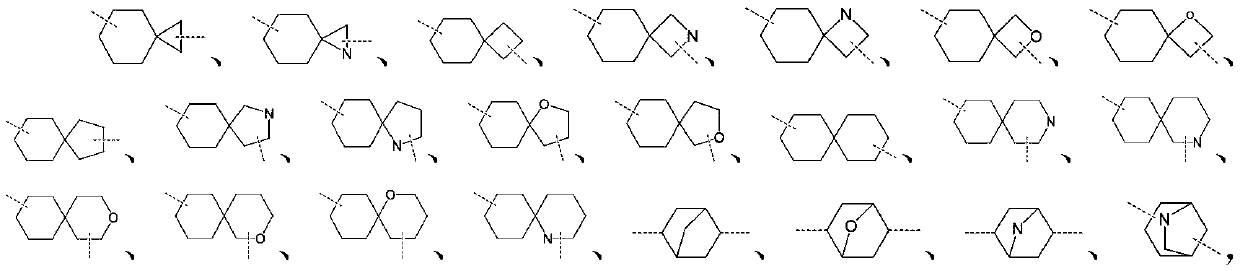
Preparation method of indoleamine 2,3-dioxygenase inhibitor
An alkoxyl, C1-C6 technology, applied in the field of preparation of indoleamine 2,3-dioxygenase inhibitors, can solve the problems of clinical activity limitation, poor biological activity of NLG919, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] N-(4-chlorophenyl)-6-(6-fluoroquinolin-4-yl)spiro[2.5]octane-1-carboxamide N-(4-chlorophenyl)-6-(6-fluoroquinolin-4-yl ) spiro[2.5]octane-1-carboxamide
[0094]
[0095]Step 1: Dissolve 1,4-cyclohexanedione monoethylene glycol ketal (10.0 g, 64.03 mmol) in 250 mL of methyl tert-butyl ether, add N-phenylbis(trifluoromethanesulfonyl ) imine (22.9 g, 64.03 mmol). The reaction solution was cooled to -78°C, and sodium bis(trimethylsilyl)amide (2 mol / L tetrahydrofuran solution) (32 mL, 64.03 mmol) was added dropwise to the reaction solution under a nitrogen atmosphere. After the dropwise addition, the reaction solution was stirred at this temperature for 60 minutes, then the reaction solution was raised to room temperature, and stirred overnight until TLC detected that the reaction raw materials were completely consumed. The reaction solution was quenched with 3 mL aqueous potassium bisulfate solution, the solid was removed by filtration, and the filtrate was concentrate...
Embodiment 2
[0104] N-(4-fluorophenyl)-6-(6-fluoroquinolin-4-yl)spiro[2.5]octane-1-carboxamide
[0105] N-(4-fluorophenyl)-6-(6-fluoroquinolin-4-yl)spiro[2.5]octane-1-carboxamide
[0106]
[0107] The synthesis of compound 2 is the same as that of compound 1, and 4-chloroaniline in Example 1 is replaced by 4-fluoroaniline. Compound 2 (16.97 mg) was obtained as a white solid with a yield of 22%. MS(ESI):m / z 393.3 (M+H) + . 1 H NMR (500MHz, d 6 -DMSO)δ10.28(s,1H),8.81(s,1H), 8.10–8.05(m,1H),8.03(d,J=11.0Hz,1H),7.69–7.60(m,3H),7.37 (s, 1H),7.13(t,J=8.0Hz,2H),3.49–3.40(m,1H),2.20(t,J=12.0Hz,1H), 1.98–1.85(m,4H),1.78( d, J=11.0Hz, 1H), 1.72(t, J=6.5Hz, 1H), 1.37–1.28(m, 1H), 1.17–1.07(m, 2H), 0.94–0.89(m, 1H).
Embodiment 3
[0109] N-(4-chlorobenzyl)-6-(6-fluoroquinolin-4-yl)spiro[2.5]octane-1-carboxamide
[0110]N-(4-chlorobenzyl)-6-(6-fluoroquinolin-4-yl)spiro[2.5]octane-1-carboxamide
[0111]
[0112] Synthesis of Compound 3 Starting from intermediate 1g in Example 1, it was prepared through the following steps:
[0113]
[0114] Step 1: Dissolve 1 g of compound (200 mg, 0.61 mmol) in 10 mL of ethanol, and add 4 mL of 2 mol / L sodium hydroxide solution. The reaction solution was heated to 50°C and reacted for 2 hours. After the reaction solution was cooled to room temperature, it was neutralized to pH=1 with 4 mol / L hydrochloric acid solution. The aqueous phase was extracted with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was separated by preparative thin-layer chromatography to obtain compound 3a (150 mg) as a white solid with a yield of 83%. MS (ESI):m / z 300.0(M+H) + . 1 H NMR (500MHz, d 6 -DMSO) δ1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com