NOVEL MULTI-ORGAN-CHIPS ESTABLISHING DIFFERENTIATION OF iPSC-DERIVED CELLS INTO ORGAN EQUIVALENTS
A technology of organelles and cells, applied in the field of new multi-organ chips
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
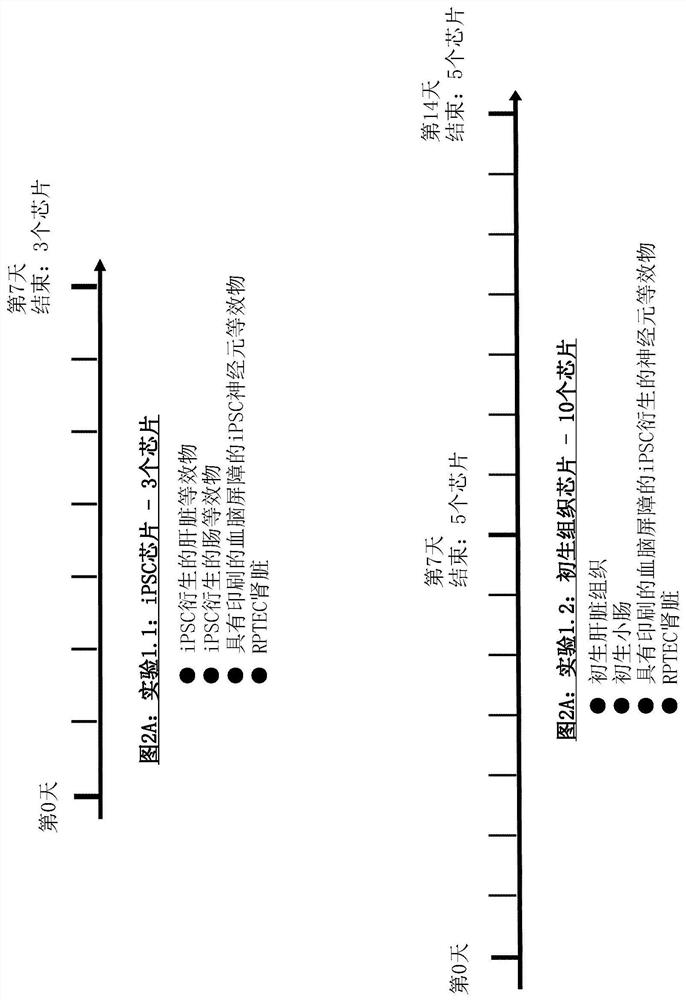
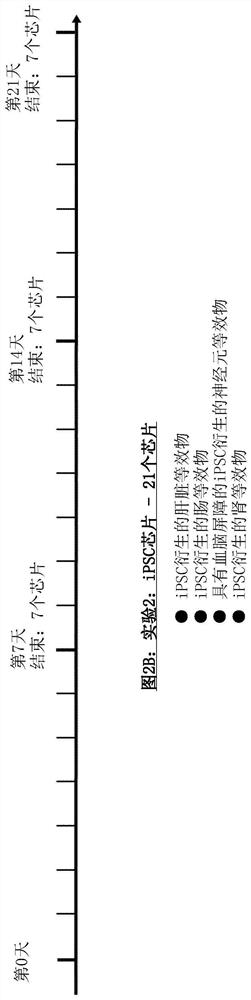
[0279] Chip Design of ADME-N Chip
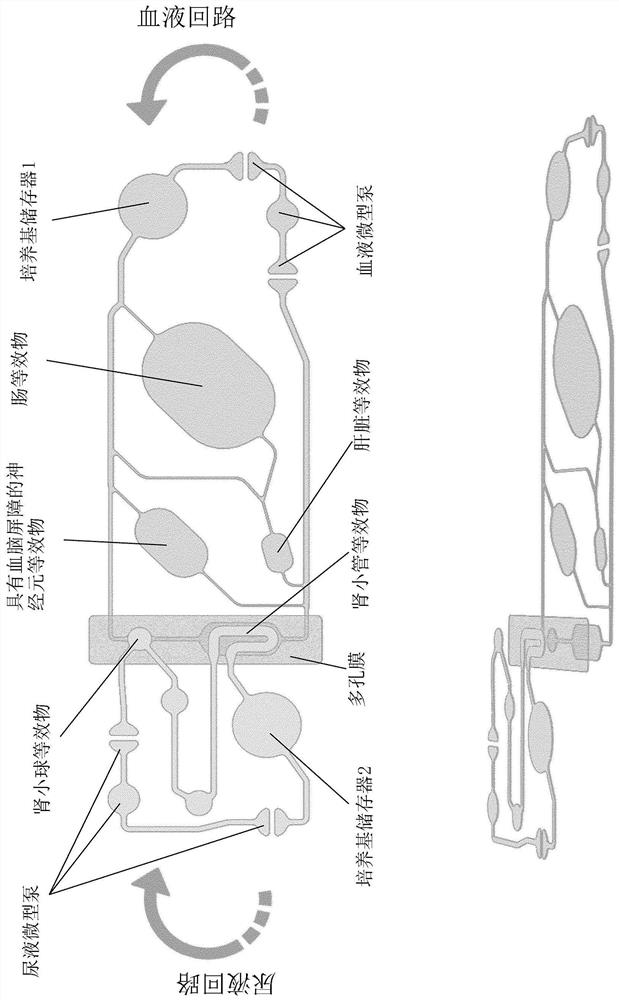
[0280] A chip design with constraints scaled down from human physiology is realized. Consider dimensional data as well as flow characteristics. The layout consists of two circuits (called "blood" and "urine") containing chambers for incorporation of the following organ equivalents: intestine, liver, kidney (divided into glomeruli and tubules) and nerve Meta organization (Figure 1). The first three tissues are used to complete the so called ADME profile (Absorption, Distribution, Metabolism, Excretion). The latter are additional tissues that complement the profile. Therefore, the chip is called ADME-N chip. One reservoir compartment per circuit allowed sampling of the supernatant (medium reservoirs 1 and 2). The medium is perfused through the microfluidic network by two built-in pneumatic micropumps (one for each circuit). The circuits overlap in two renal compartments separated by a porous membrane made of polycarbonate.
[0281] inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com