In vivo gene therapy using delivery of a lentiviral gene construct
A lentiviral vector and delivery technology, used in gene therapy, medical materials derived from viruses/phages, genetic engineering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0126] Example 1. Improving the therapeutic efficacy of in vivo platelet-targeted gene therapy for hemophilia A
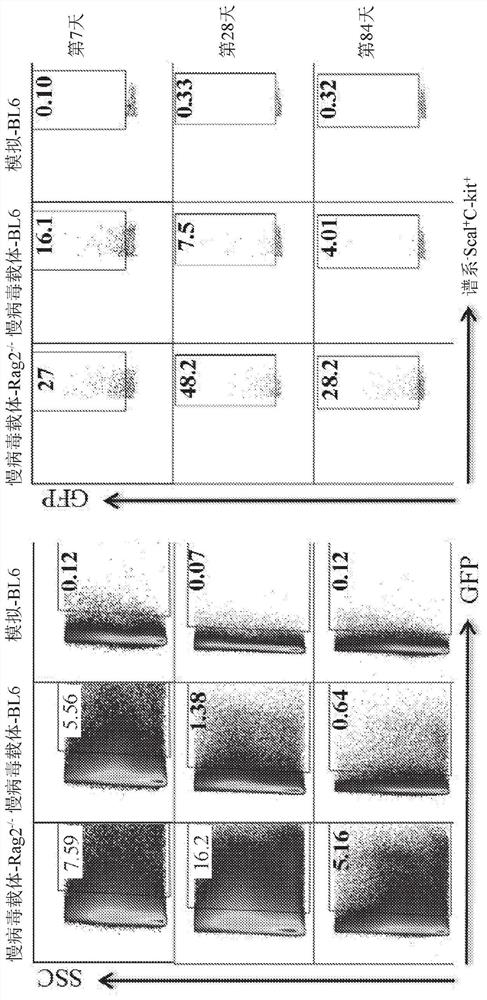
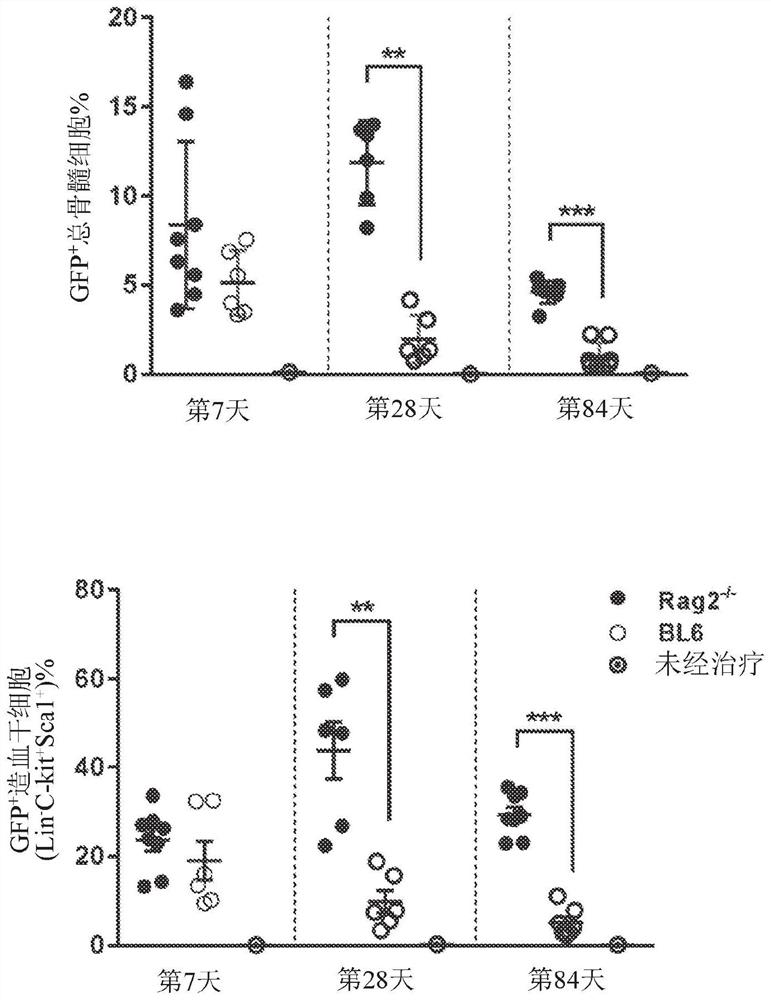
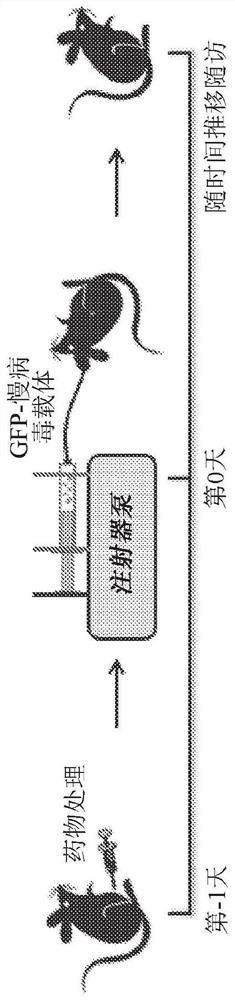
[0127] A previously described in vivo hematopoietic stem cell (HSC)-based platelet-targeted gene therapy protocol partially corrected the bleeding phenotype in hemophilia A (HemA) mice over five months. In the described protocol, a lentiviral vector (LV) carrying a factor III (FVIII) transgene (G-F8 / N6-LV) driven by a megakaryocyte-specific promoter (Gp1bα) is delivered by intraosseous (IO) infusion to transduce HSCs. Maturation of HSCs transduced along the megakaryocyte lineage leads to ectopic FVIII production in platelets. However, there is still a need for an effective and clinically feasible protocol to improve LV transduction efficiency and increase platelet-FVIII function.
[0128] To enhance LV transduction, this example describes a combination drug regimen of dexamethasone and anti-CD8α monoclonal antibody, followed by IO infusion of LV in mice. In M-GF...
example 2
[0178] Example 2. Intraosseous delivery of lentivirus as a therapeutic strategy for in vivo gene therapy for hemophilia
[0179] As described above, lentiviral vectors (LV) delivered by intraosseous (IO) infusion at precisely controlled rates can efficiently transduce bone marrow hematopoietic stem cells (HSCs) in mice. Infusion of LV IO carrying a human FVIII / N6 transgene driven by a platelet-specific Gp1bα promoter into hemophilia A (HemA) mice produced FVIII stored in platelet alpha granules. These platelet FVIII partially corrected the bleeding phenotype over five months in HemA mice with or without pre-existing anti-FVIII inhibitors.
[0180] In the present example, LV IO delivery was applied to humanized NSG mice to establish a translational research model for in vivo gene therapy in hemophilia. First, it was examined whether high levels of transgene expression could be achieved in human megakaryocytes (Meg). Human CD34 was transduced with Cocal-MND-GFP-LV (M-GFP-LV, M...
example 3
[0185] Example 3. Intraosseous delivery of lentivirus produces human platelet-specific Factor VIII in humanized NSG mice
[0186] This example establishes a proof-of-principle research model in humanized NSG mice for the translational application of this novel strategy in the human clinic.
[0187] Hemophilia A (HemA) lacking functional plasma factor VIII (FVIII) is an ideal candidate disease for gene therapy to achieve long-term therapeutic FVIII levels.
[0188] This article describes a new clinically translatable strategy for the treatment of HemA. In this strategy, a self-inactivating lentiviral vector (LV) carrying a FVIII transgene driven by the platelet-specific promoter Gp1bα(G) (G-FVIII-LV) was passed intraosseously ( 10) Administration delivery into HemA mice. G-FVIII-LV can effectively transduce hematopoietic stem cells (HSC). Then, FVIII specifically expressed and stored in platelet α-granules could partially correct the bleeding phenotype over five months in im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com