Dietary fiber for treating patients suffering from methylmalonic acidemia and propionic acidemia
A technology of methylmalonic acidemia and dietary fiber, applied in the direction of acid-containing food ingredients, applications, food science, etc., can solve problems such as inability to infer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
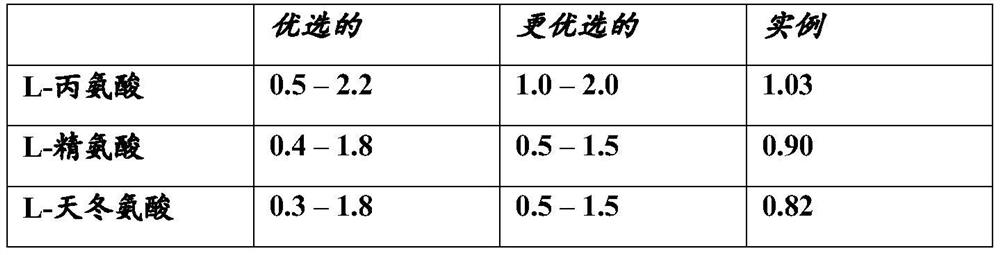
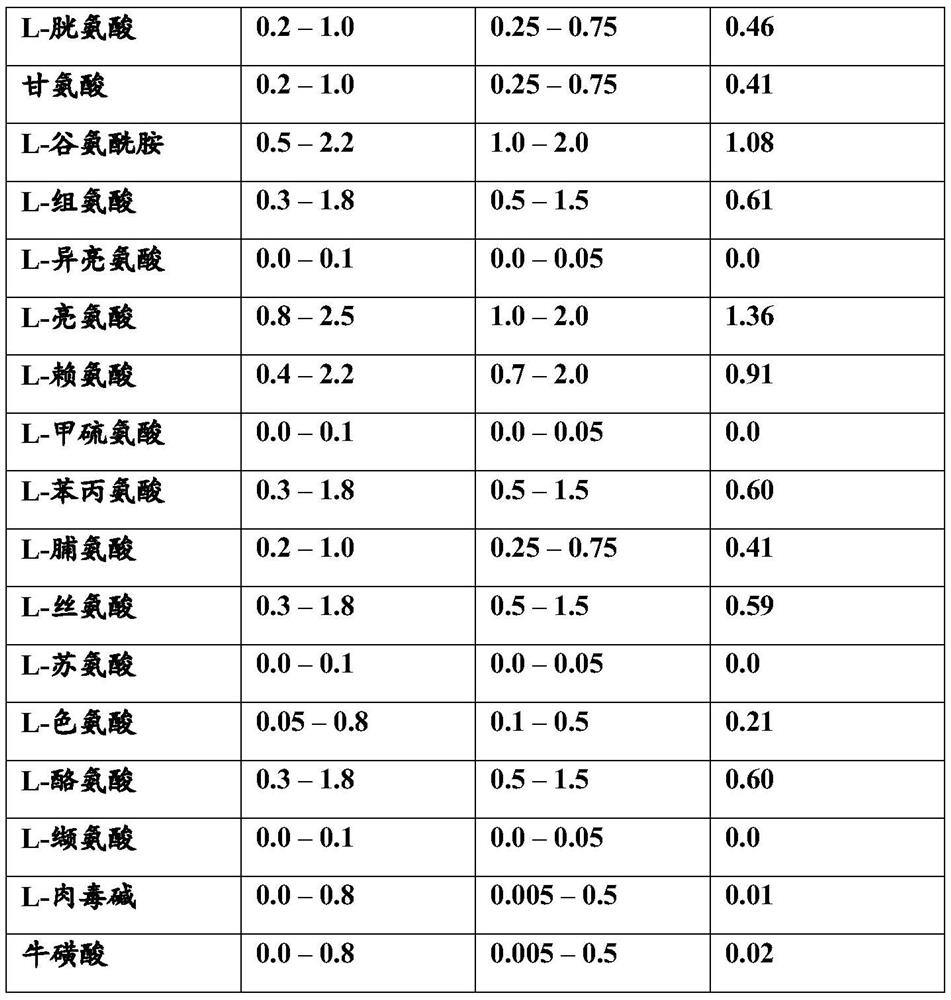
Examples
Embodiment 1
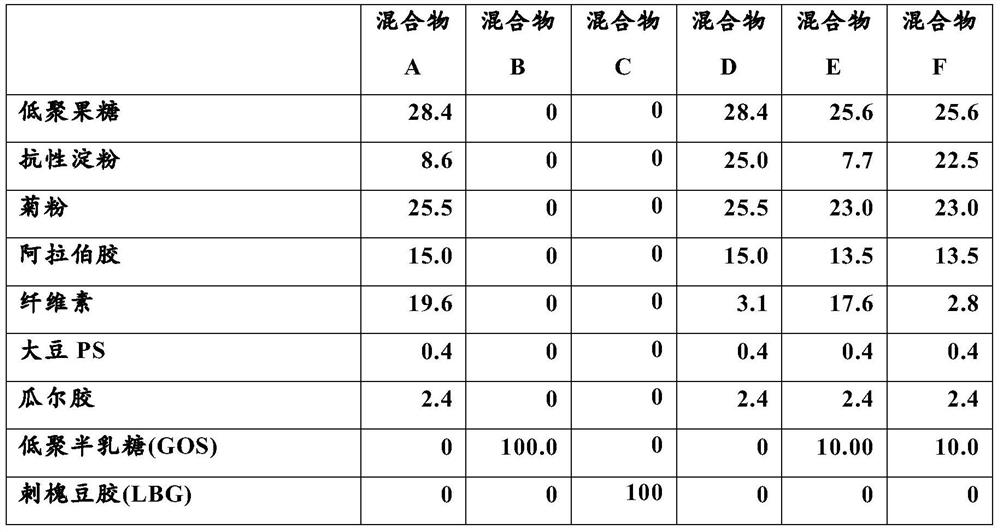
[0109] fermentation
[0110] In the high-throughput anaerobic colon model (I-screen TM Platform, TNO, Zeist, Netherlands) after incubation for 24 hours, the effect of the fiber mixtures in Table 1 on SCFA production was measured. The platform mimics gut microbiota conditions and enables the determination of the effects of compounds on microbiota composition over time under strictly anaerobic conditions. Inoculate I-screen with standard healthy human adult gut flora TM Model. Gut microbiota were obtained by pooling stool samples from 6 healthy adult volunteers and incubating the pooled stool samples in fed-batch fermenters for 40 h to generate standardized microbiota. The pooled stool samples were then cultured in vitro in modified standard ileal efflux medium (SIEM) with pH adjusted to 5.8. This standard adult gut flora was stored at -80°C in 12% glycerol until further analysis. This pooled approach aims to limit inter-individual variability and increase the likelihood ...
Embodiment 2
[0123] Example 2: Compositions of the invention
[0124] The following compositions of the present invention are compositions suitable for the treatment of MMA and PA by modulating intestinal flora such that propionic acid production is reduced. The supplement comprises the following ingredients in % by weight, based on the total weight of the composition:
[0125] 1.5-28.4% fructooligosaccharides
[0126] 1.5-25.5% inulin
[0127] 1.5-50% resistant starch
[0128] 1-15% gum arabic
[0129] 0-0.5% soybean polysaccharide
[0130] 0-3% guar gum
[0131] ·5-79.5% galacto-oligosaccharides
[0132] • Optionally supplemented with lactic acid producing bacteria, preferably Bifidobacteria and / or Lactobacilli.
Embodiment 3
[0133] Example 3: Compositions of the invention
[0134] The following compositions of the present invention are compositions suitable for the treatment of MMA and PA by modulating intestinal flora such that propionic acid production is reduced. The supplement comprises the following ingredients in % by weight, based on the total weight of the composition:
[0135] ·5-100% galacto-oligosaccharide
[0136] 0-70% resistant starch
[0137] • Optionally supplemented with lactic acid producing bacteria, preferably Bifidobacteria and / or Lactobacilli.
[0138] • Optionally a source of lipids and a source of digestible carbohydrates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com