Combination of IAP inhibitors with PARP or MEK inhibitors or other chemotherapeutic agents
A technology of inhibitors and compositions, applied in the direction of drug combination, organic chemistry, anti-toxic agent, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0221] abbreviation
[0222]
[0223]
[0224]
[0225] compound synthesis
[0226] The preparation of the IAP inhibitors of the present invention is disclosed in International Patent Published Application No. WO2014 / 031487, which is incorporated herein by reference in its entirety.
example 1
[0228] animal:
[0229] Balb / c nude mice, female, 4-6 weeks old, weighing about 18-22 g, were purchased from GemPharmatech Co., Ltd (Nanjing, Jiangsu).
[0230] Biogenesis of Xenograft (PA1170, PA6265, PA0787, and PA1194) Models Derived from Subcutaneous Pancreatic Cancer Patients to make:
[0231] All PDX models were initially established from surgically resected clinical samples (cancer type: pancreatic adenocarcinoma) and implanted into nude mice defined as passage 0 (P0). The next passage from P0 tumor implantation was defined as passage 1 (P1), and so on during serial implantation of mice. Frozen tumor fragments were revived in NOD SCID mice, and when tumors had grown to an appropriate size, they were passaged to Balb / c nude mice. P4-P7 tumors were used for research. Complete exome sequencing and RNA sequencing revealed BRCA2, CDKN2A, and KRAS mutations in PA1170; BRCA1, KRAS, and TP53 mutations in PA6265; CDKN2A, KRAS, and TP53 mutations in PA0787; and KRAS and ...
example 24
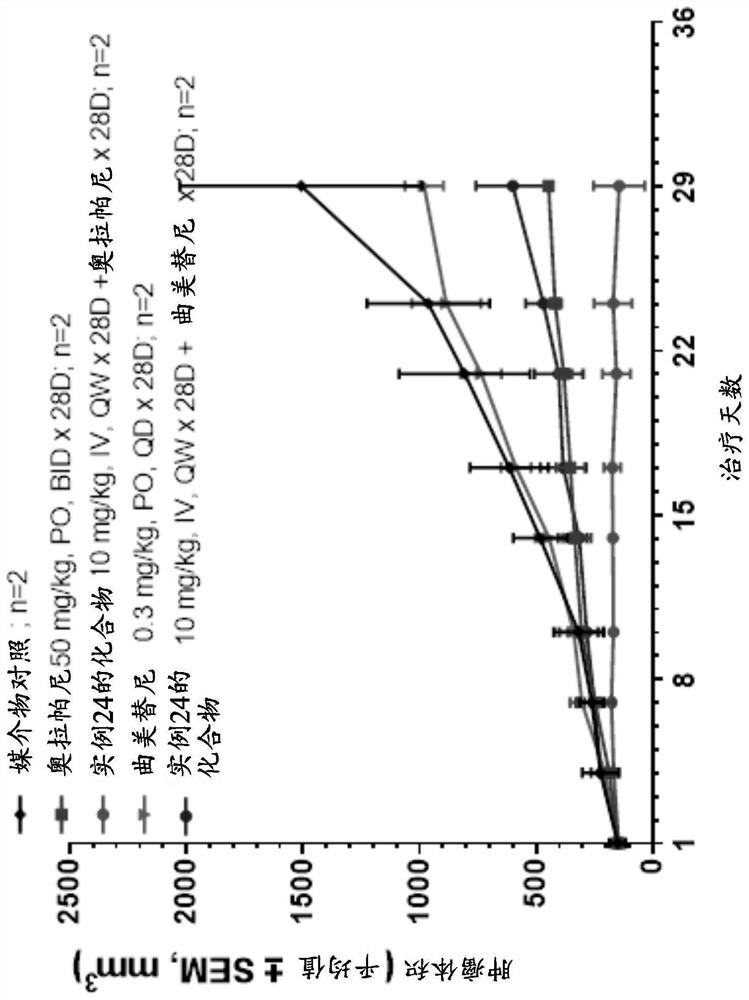
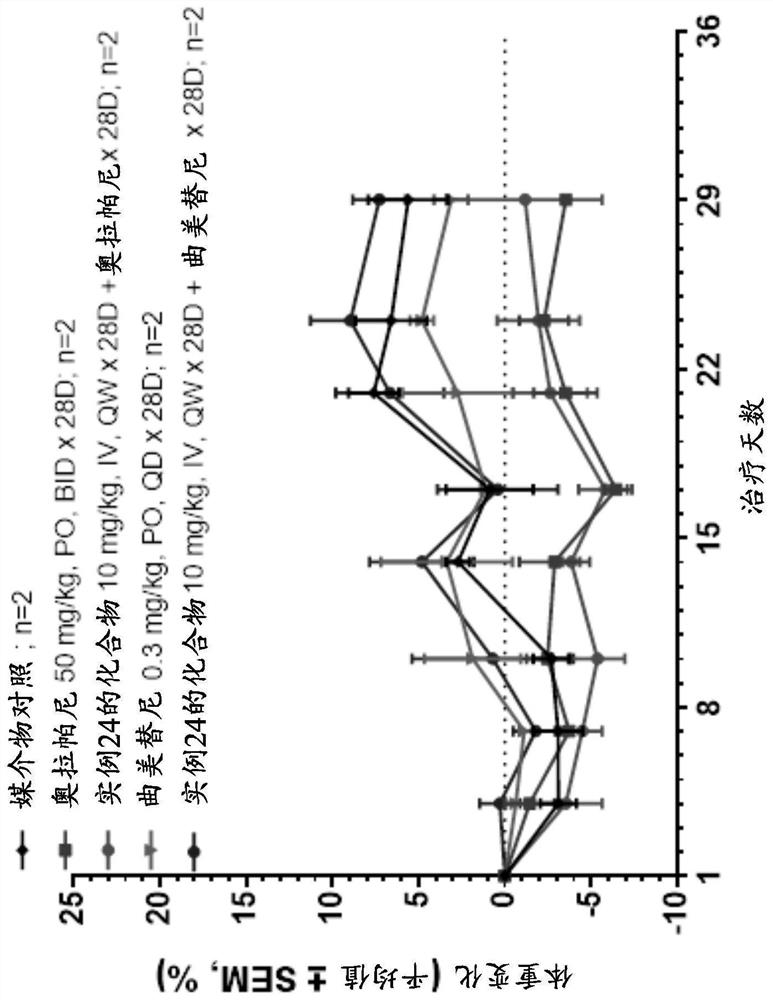
[0267] The antitumor activity of the compound of Example 24 plus olaparib in PDX model PA6265 of pancreatic cancer carrying BRCA1 mutation is shown in image 3 and Table 4, olaparib was moderately active on tumor growth at 50 mg / kg (p.o. BID for 4 weeks), with a T / C value of 72% at day 28 (P Figure 4 As shown, there was no significant change in body weight during all treatment periods in PA7265.
[0268] Table 4 RTV, RTV, T / C (%) value and synergy score at key time points in PA6265
[0269]
[0270]
[0271] Taken together, these results indicate that the combination therapy of the compound of Example 24 and olaparib achieves a synergistic effect and further enhances the anti-tumor effect in tumors carrying BRCA1 or BRCA2 mutations.
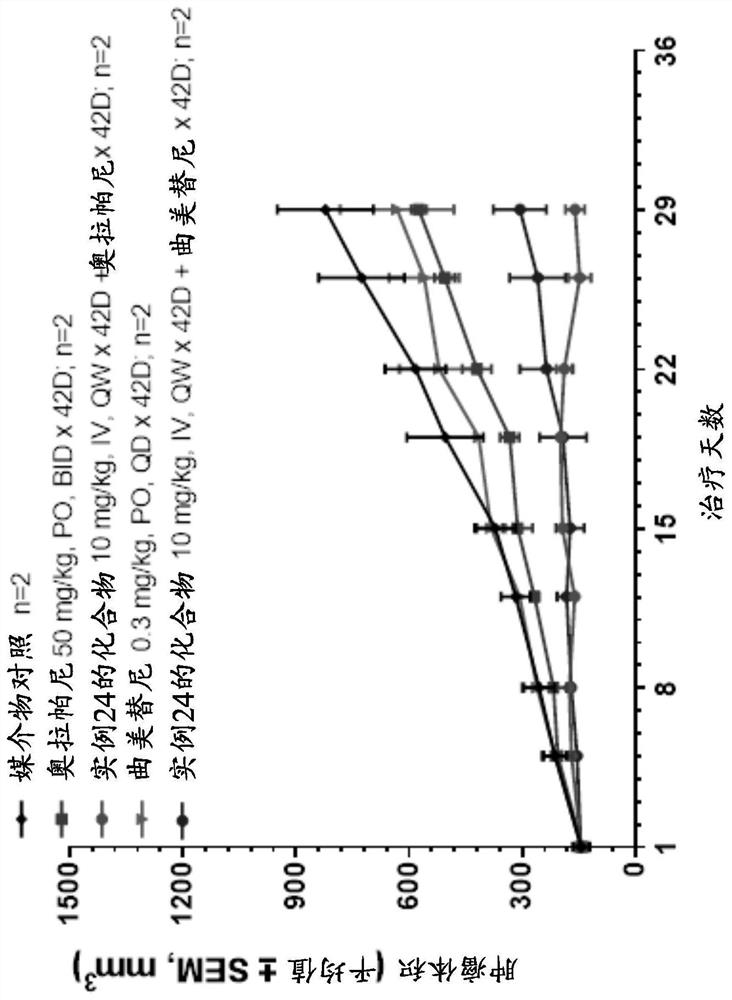
[0272] B. The compound of Example 24 improves the antitumor activity of trametinib in a pancreatic PDX model.
[0273] The compound of Example 24 was tested in combination with Trametinib in xenograft models PA1170 and PA6265 derived from ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap