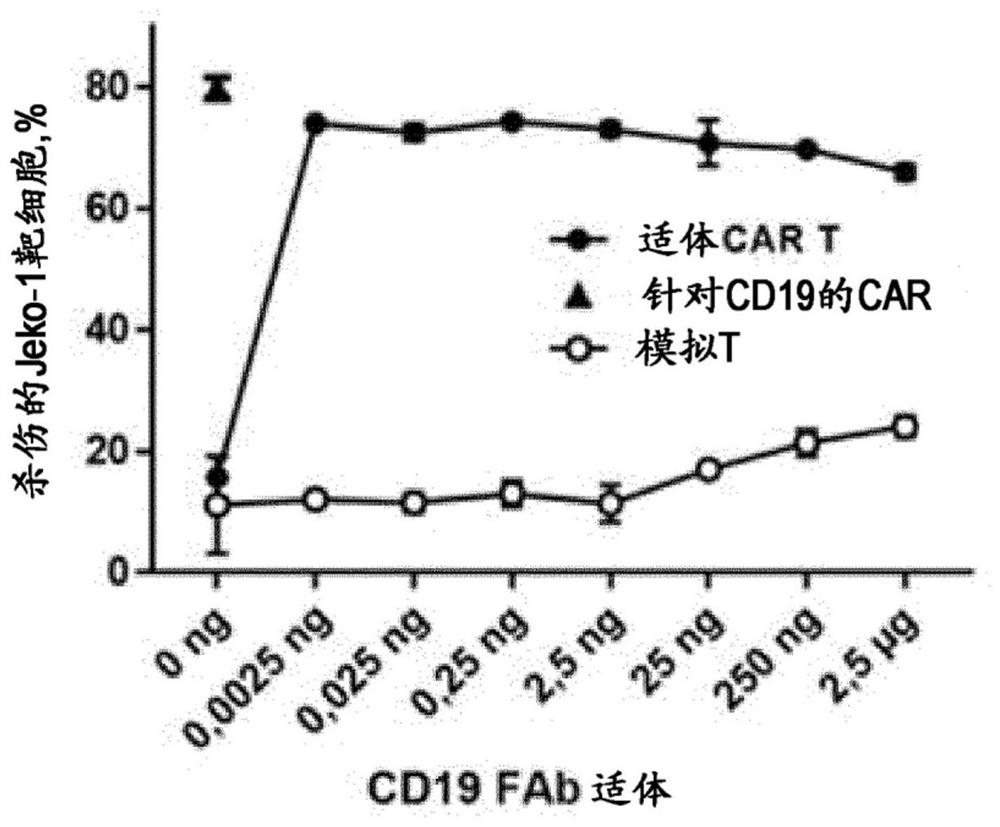
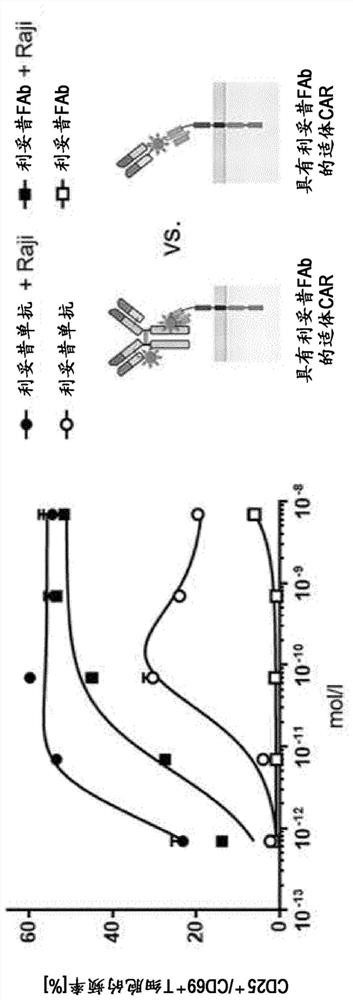
A combination of compositions for elimination and enhanced engraftment of hematopoietic stem cells in the bone marrow of a subject
A technology of hematopoietic stem cells and compositions, which can be used in drug combinations, extracellular fluid diseases, medical raw materials derived from mammals, etc., and can solve problems such as intolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
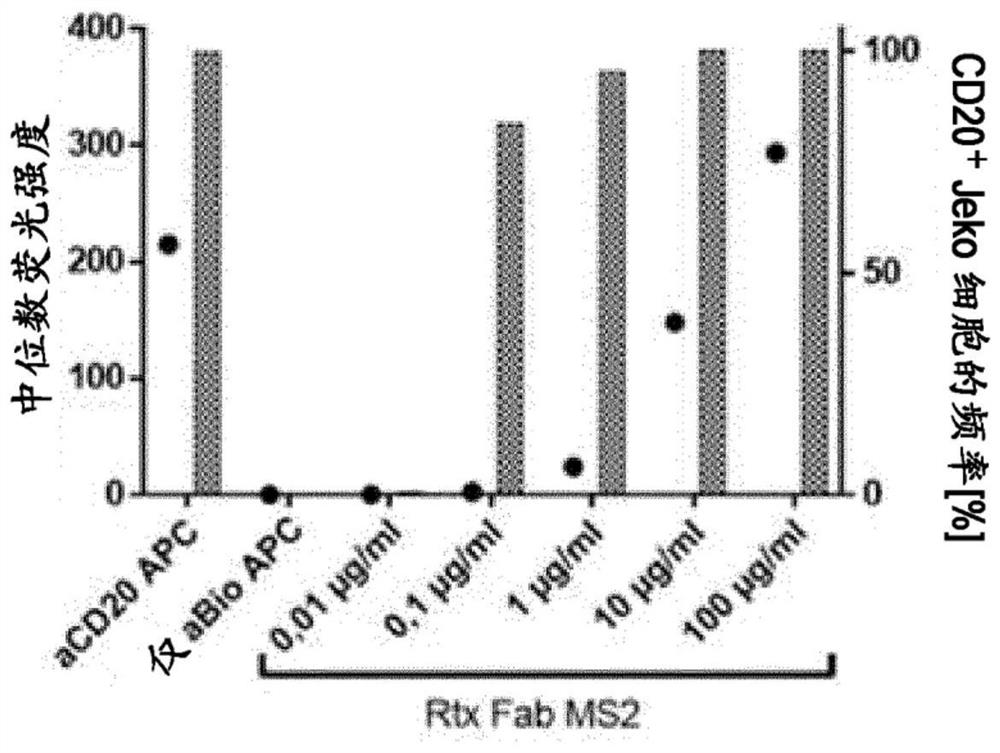
[0177] Example #1: Binding to CD20 Positive Jeko 1 The antigen recognition portion Z of the CD20 antigen on the cell (CD20 Function of Fab) conjugated and stained with fluorescent anti-biotin APC biotin-crosslinker YCD20 positive Jeko-1 cells were seeded in 96-well plates (50.000 cells / well) and conjugated with biotin-crosslinker at different concentrations (0,01-100 μg / ml and 0 μg / ml as negative control) Anti-CD20 Fab (Rtx Fab MS2) was incubated in a total volume of 50 μl buffer A (CliniMACS PBS / EDTA buffer + 0.5% BSA) for 10 min at 4°C. After the addition of 50 μl Antibiotin APC (1:50 in Buffer A, Miltenyi Biotec, Art. No. 130-110-952), the samples were incubated for a further 10 min at 4°C. Anti-human CD20 APC conjugate as a positive control was used to stain control samples according to the manufacturer's protocol (Miltenyi Biotec, Art. No. 130-111-525). Finally, 100 μl of buffer A was added to each sample and data was collected on MACSQuant Analyzer 10 (Miltenyi Bio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com