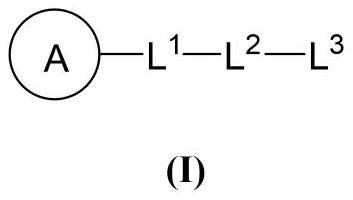
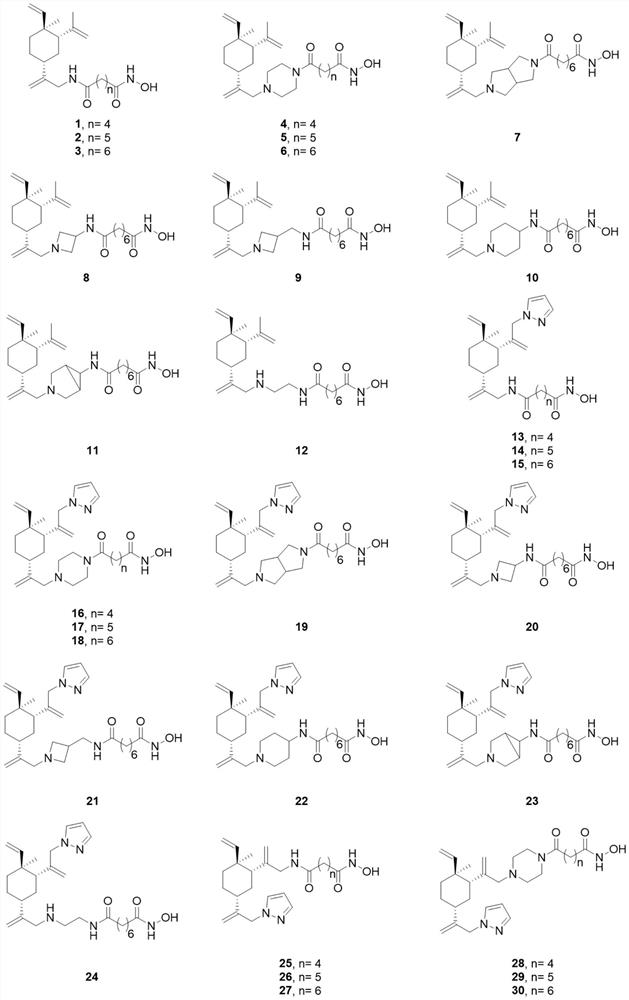
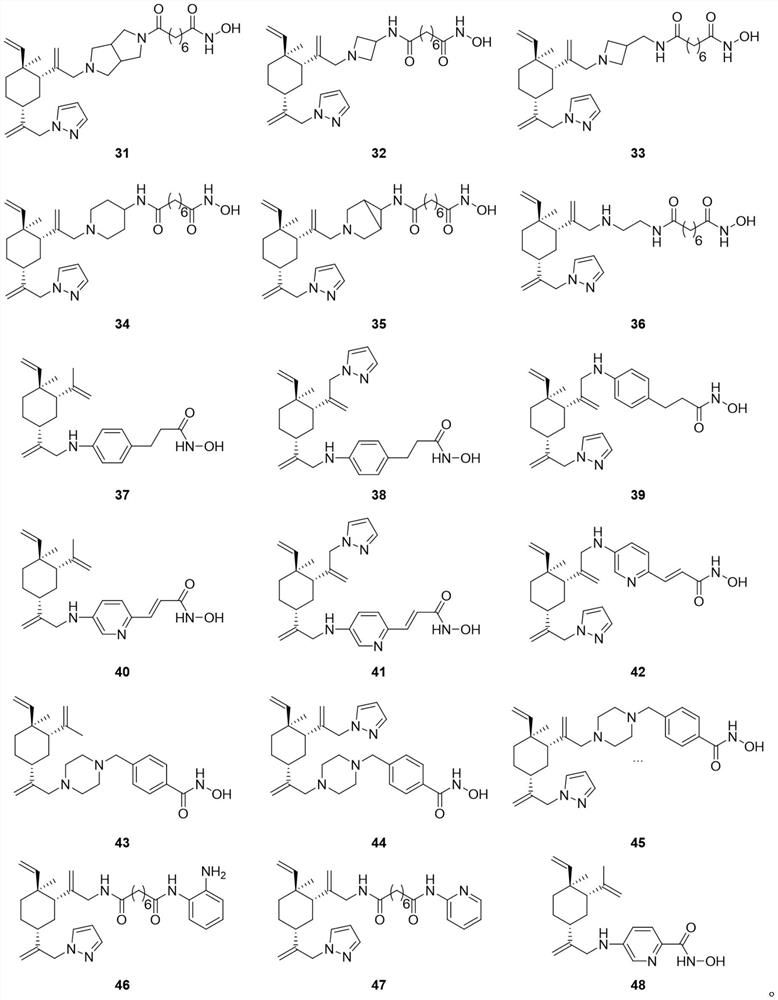
Beta-elemene derivative with HDACi pharmacophore as well as preparation method and application of beta-elemene derivative
A derivative and pharmacophore technology, applied in the field of beta-elemene derivatives and their preparation, can solve the problems of designing and synthesizing a single anti-tumor molecule, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of Compound 1
[0067]
[0068] Intermediate 1a
[0069] To a solution of β-elemene (210 mg, 1.029 mmol) in acetic acid (3 mL) under ice bath was added N-bromosuccinimide (NBS, 183 mg, 1.029 mmol). Stir under ice bath condition for 6h. TLC monitoring that the raw materials were not completely reacted, so the reaction solution was warmed to room temperature and stirred overnight (25-28°C). After complete conversion, saturated sodium bicarbonate solution was slowly added dropwise to the reaction solution to terminate the reaction, followed by extraction with ethyl acetate (3×5 mL). The combined organic phases were washed with saturated brine (2×5 mL) and dried over anhydrous sodium sulfate. The drying agent was removed by filtration, the filtrate was concentrated under reduced pressure, and the obtained crude product was purified by silica gel column chromatography (eluent: n-hexane) to obtain colorless oily liquid 1a (72 mg, yield 25.4%). 1 ...
Embodiment 2
[0080] Example 2: Preparation of Compound 2
[0081]
[0082] Referring to the synthesis method of intermediate 1d in Example 1, compound 2b (252 mg, yield 70.9%) was obtained.
[0083] Referring to the synthesis method of intermediate 1e in Example 1, compound 2c (130 mg, 80.1%) was obtained.
[0084] Referring to the synthesis method of the last step in Example 1, colorless oily liquid 2 was obtained, yield: 58.9%. 1 HNMR (500MHz, CDCl 3 )δ6.35(s, 1H), 5.80(dd, J=17.7, 10.5Hz, 1H), 4.95-4.87(m, 3H), 4.86(s, 1H), 4.82(t, J=1.7Hz, 1H) ),4.57(d,J=1.9Hz,1H),3.85(d,J=5.6Hz,2H),2.29-2.11(m,4H),2.04-1.90(m,2H),1.70(s,3H) , 1.68-1.53(m, 5H), 1.52-1.40(m, 3H), 1.40-1.24(m, 4H), 0.99(s, 3H). 13 C NMR (126MHz, CDCl 3 )δ173.67,171.44,150.52,149.99,147.40,112.27,110.06,108.32,52.67,43.00,42.72,39.85,39.77,36.16,33.11,32.36,27.20,25.08,16.6.89,244 LCMS[M+H] + :377.2.
Embodiment 3
[0085] Example 3: Preparation of Compound 3
[0086]
[0087] Referring to the synthesis method of intermediate 1d in Example 1, compound 3b (216.4 mg, yield 77%) was obtained. 1 HNMR (400MHz, CDCl 3 )δ8.90(s,1H),4.95(s,1H),3.97(t,J=10.2Hz,1H),3.65(dd,J=10.9,5.4Hz,1H),2.34(t,J=7.4 Hz, 2H), 2.12 (t, J=7.4Hz, 2H), 1.91-1.74 (m, 3H), 1.63 (ttd, J=18.5, 13.0, 11.4, 5.6Hz, 7H), 1.35 (p, J= 3.9Hz, 4H).
[0088] Referring to the synthesis method of intermediate 1e in Example 1, compound 3c (140 mg, 80.1%) was obtained.
[0089] Referring to the synthesis method of the last step in Example 1, the difference is that the obtained crude product was purified by silica gel column chromatography (methanol:dichloromethane=1:9) to obtain rust red oily compound 3 (59.6 mg, yield 72.3%) ). 1 H NMR (500MHz, CDCl 3 )δ6.35(s,1H),5.80(dd,J=17.7,10.5Hz,1H),5.00-4.79(m,5H),4.57(s,1H),3.85(d,J=5.6Hz,2H ), 2.23(t, J=7.2Hz, 2H), 2.16(s, 2H), 2.05-1.89(m, 2H), 1.70(s, 3H), 1.69-1.22(m, 14H), 0....
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap