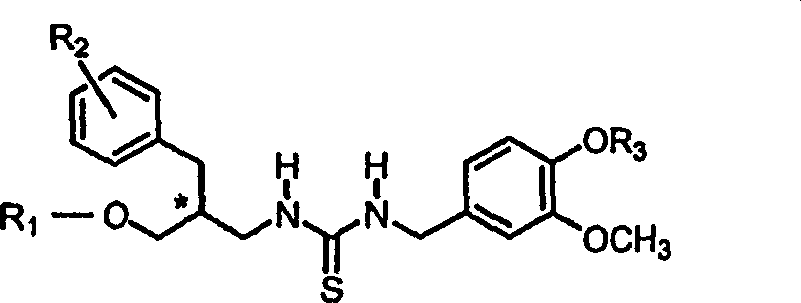
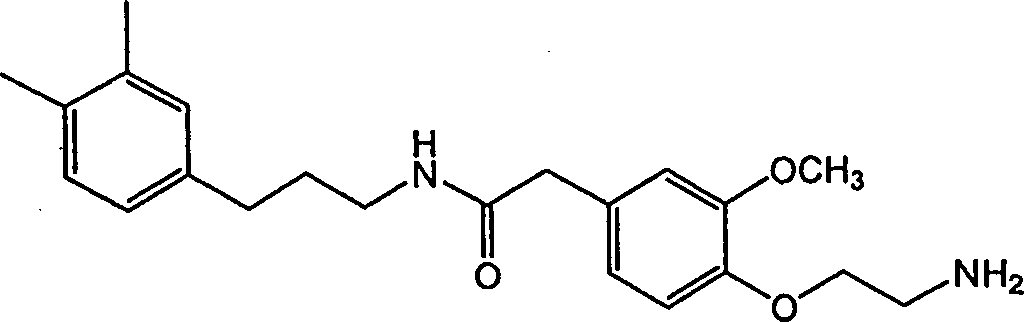
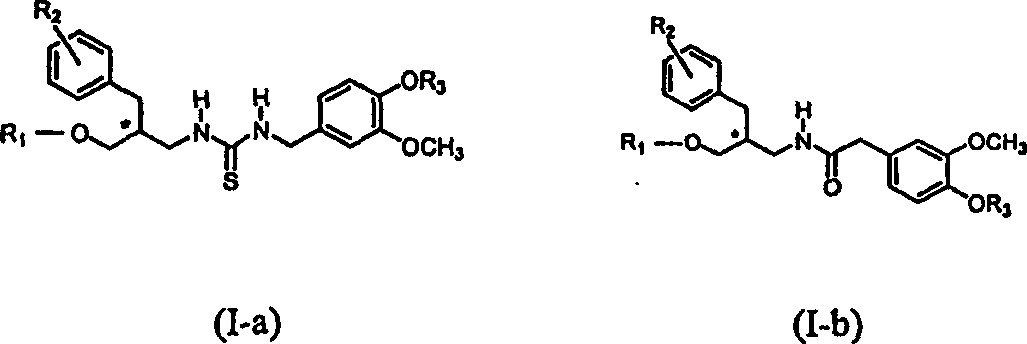
Vanilloid analogues containing resiniferatoxin pharmacophores as potent vannilloid receptor agonists and analgesics, compositions and uses thereof
A group and drug technology, applied in the field of vanilla analogs, can solve the problems of low efficiency, complex structure, difficult synthesis and acquisition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] A cold solution of diethyl malonate (6.4 g, 40 mmol) in DMF (20 mL) at 0 °C was treated stepwise with sodium hydride (60%, 1.92 g, 48 mmol) and stirred at room temperature for 40 min. The reaction mixture was treated with the corresponding substituted benzyl chloride (48 mmol) and stirred overnight at room temperature. The reaction mixture was diluted with water and extracted several times with EtOAc. Combine the organic layers, wash with water and brine, wash with MgSO 4 Dry and concentrate in vacuo. Flash column chromatography of the residue on silica gel with EtOAc / hexane (1:10) as eluent afforded 1. Example 1: Diethyl 3,4-dimethylbenzylmalonate (1b)
[0079] 70% yield, colorless oil; 1 H NMR (CDCl 3 )δ6.9-7.05 (m, 3H), 4.14 (m, 4H, 2×CO 2 CH 2 ), 3.61(t, 1H, CH), 3.25(d, 1H, CH 2 Ar), 3.14(d, 1H, CH 2 Ar), 2.2-2.25(m, 6H, 2×CH 3 ), 1.21(m, 6H, 2×CO 2 CH 2 CH 3 ) Example 2: Diethyl 4-chlorobenzylmalonate (1c)
[0080] 74% yield, colorless oil; 1 H NMR ...
Embodiment 4
[0083] 98% yield, white solid, mp=67°C; 1 H NMR (CDCl 3 ) δ7.15-7.30 (m, 5H, phenyl), 3.74 (dd, 2H, CH 2 OH), 3.62 (dd, 2H, CH 2 OH), 2.9-3.0 (bs, 2H, OH), 2.58 (d, 2H, CH 2 Ph), 2.03 (m, 1H, CH). Example 5: 2-(3,4-Dimethylbenzyl)-1,3-propanediol (2b)
Embodiment 5
[0084] 92% yield, colorless oil; 1 H NMR (CDCl 3 ) δ6.88-7.06 (m, 3H, phenyl), 3.6-3.8 (m, 4H, 2×CH 2 OH), 2.5-2.7 (bs, 2H, OH), 2.60 (d, 1H, CH 2 Ph), 2.52(d, 1H, CH 2 Ph), 2.2-2.28(m, 6H, 2×CH 3 ), 2.02 (m, 1H, CH). Example 6: 2-(4-chlorobenzyl)-1,3-propanediol (2c)
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap