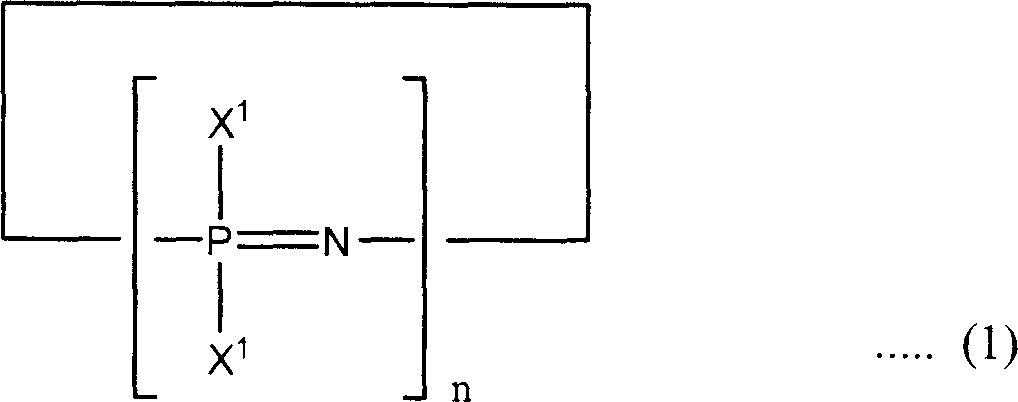
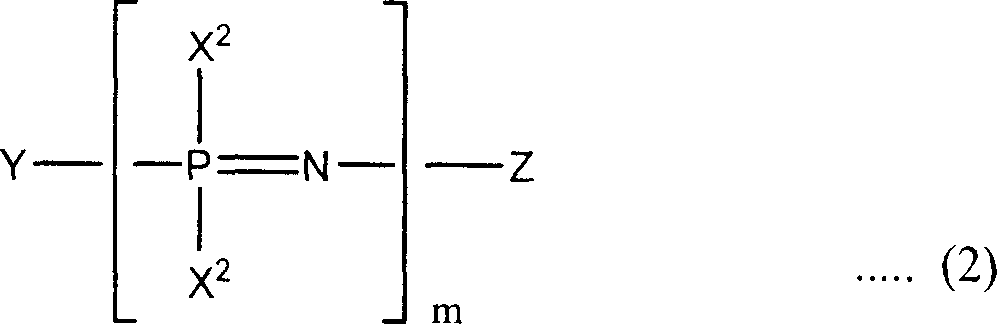
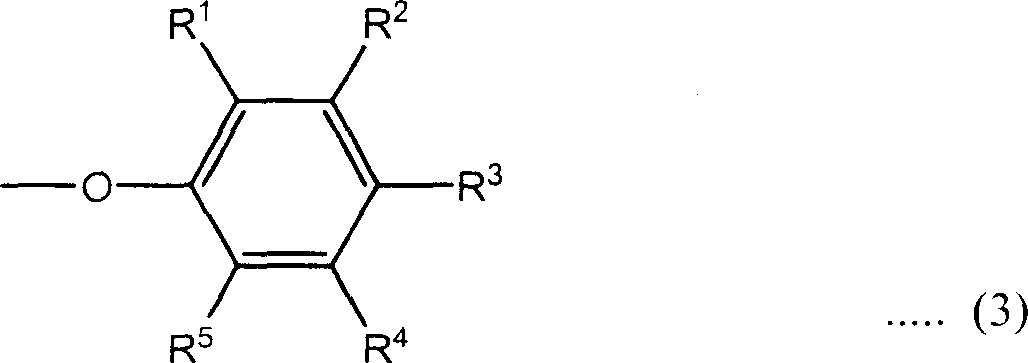
Phosphazene composition
A technology for phosphazene compounds and compositions, applied in the field of flame retardants, flame retardant resin compositions, and phosphazene compositions, can solve the problem of insufficient hydrolysis resistance and electrical stability of phosphazene compositions, and no mention of volatility. Component content flame retardancy, hydrolysis resistance and stability of electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] FR1: Add 160.2g of phenol, 112.2g of solid potassium hydroxide and 500ml of xylene to a 2L four-necked flask equipped with a Dimroth condenser, a Dean-Stark tube, a dropping funnel, a thermometer and a stirrer under nitrogen. The stream was heated to reflux at an oil bath temperature of 145°C. The produced water is removed from the system by azeotroping with xylene, and only xylene is returned to the system. Heating to reflux was carried out until distillation of the water produced ceased. The reaction takes 4 hours to complete.
[0114] The reaction vessel was immersed in an ice bath and cooled until the reaction mixture reached 10°C or lower, then 72.1 g of chlorophosphazene was deionized in 30 minutes using a dropping funnel while maintaining the reaction mixture at 10°C or lower. A mixed solution of the polymer (trimer: 95%, tetramer: 4%, other components: 1%) and 250 ml of xylene was added dropwise to the reaction mixture. After adding the mixed solution, the re...
Embodiment 2
[0116] FR2: Add 151.5g of phenol, 103.6g of solid potassium hydroxide and 500ml of xylene into a 2L four-necked flask equipped with a Dimroth condenser, a Dean-Stark tube, a dropping funnel, a thermometer and a stirrer and place under nitrogen The stream was heated to reflux at an oil bath temperature of 145°C. The produced water is removed from the system by azeotroping with xylene, and only xylene is returned to the system. Heating to reflux was carried out until distillation of the water produced ceased. The reaction takes 4 hours to complete.
[0117] The reaction vessel was immersed in an ice bath and cooled until the reaction mixture reached 10°C or lower, then 70.0 g of chlorophosphazene was deionized in 30 minutes using a dropping funnel while maintaining the reaction mixture at 10°C or lower. A mixed solution of the polymer and 250 ml of xylene was added dropwise to the reaction mixture. After adding the mixed solution, the reaction mixture was heated again and hea...
Embodiment 3
[0119] FR3: Add 158.0g of phenol, 110.0g of solid potassium hydroxide and 500ml of chlorobenzo into a 2L four-necked flask equipped with a Dean-Stark tube with a Dimroth condenser, dropping funnel, thermometer and stirrer and dissolve under nitrogen The stream was heated to reflux at an oil bath temperature of 145°C. The water produced is removed from the system by azeotroping with chlorobenzene, and only chlorobenzene is returned to the system. Heating to reflux was carried out until distillation of the water produced ceased. The reaction takes 6 hours to complete.
[0120] The reaction vessel was immersed in an ice bath and cooled until the reaction mixture reached 10°C or lower, then 72.1 g of chlorophosphazene was deionized in 30 minutes using a dropping funnel while maintaining the reaction mixture at 10°C or lower. A mixed solution of polymer and 250 ml of chlorobenzene was added dropwise to the reaction mixture. After adding the mixed solution, the reaction mixture w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap