Process for the preparation of fexofenadine
A technology based on dimethylphenylacetic acid and cyclopropyl ketone, which is applied in the field of intermediates for the preparation of the antihistamine fexofatine, and can solve problems such as difficulties in commercial application, cumbersome processes, and reduced yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
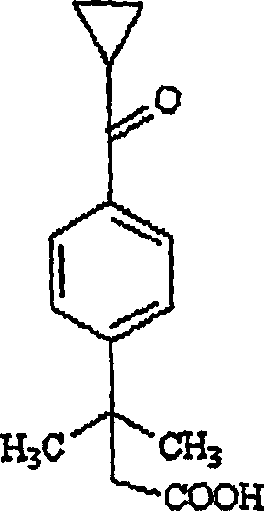
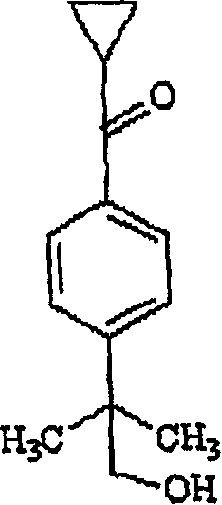
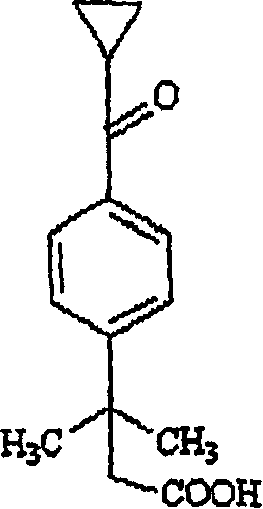
[0022] The inventors have developed an efficient method for the preparation of cyclopropylketo α,α-dimethylphenylacetic acid by treating 4-[cyclopropylcarbonyl]-2,2-dimethylphenylacetic acid with an alkali metal hydroxide Phenylethyl alcohol, after adding an oxidizing agent, reacts in an acidic aqueous solution; then separates the cyclopropyl ketone group α, α-dimethylphenylacetic acid.
[0023] Generally, an alkali metal hydroxide solution can be prepared by dissolving the alkali metal in water, and treating 4-[cyclopropylcarbonyl]-2,2-dimethylphenethanol with the solution. In addition, the solution can be prepared in a solvent if the alkali metal hydroxide is soluble, including, for example, lower alkanols, ketones, water and mixtures thereof.
[0024] Alkali metal hydroxides include any hydroxide including, for example, lithium hydroxide, sodium hydroxide, and potassium hydroxide.
[0025] Usually, the reaction of 4-[cyclopropylcarbonyl]-2,2-dimethylphenethanol and alkali ...
Embodiment
[0037] Embodiment: the preparation of cyclopropyl keto group α, α-dimethylphenylacetic acid
[0038] To an aqueous solution of sodium hydroxide (11.5 g) was added 4-[cyclopropylcarbonyl]-2,2-dimethylphenylethanol (125 g) at room temperature to obtain a suspension. At room temperature, solid potassium permanganate was added to the suspension in small batches within 4 to 5 hours. After the reaction was complete, acetone (1 mL) was added, the manganese dioxide formed was filtered, and the filtrate was washed with dichloromethane (25 mL+12.5 mL) to remove non-acidic impurities. After the aqueous layer was acidified with hydrochloric acid, the product was isolated to give 23.7 g of high purity product.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap