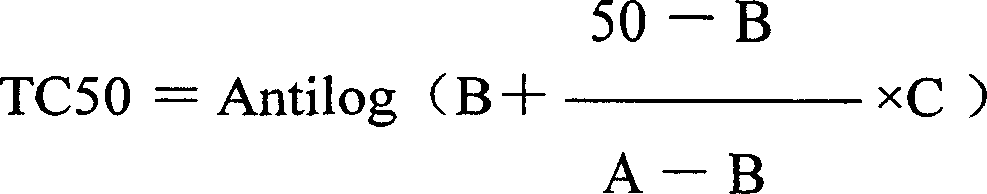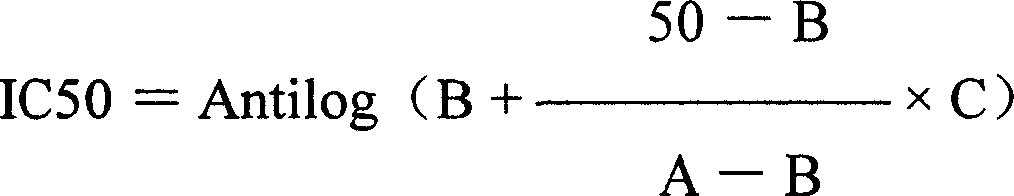Medicine composition and method for treating hepatitis with arginase
A technology of arginase and arginine deiminase, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of unsatisfactory curative effect and adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation of embodiment 1 test material
[0020] 1.1 Test drug
[0021]Name: Pegylated recombinant human arginase (BCT-100), hereinafter referred to as "arginase". The enzyme has the nucleotide sequence shown in Figures 1A, 1B and 1C and its deduced amino acid sequence.
[0022] Preparation method: Please refer to Examples 1-8 in the specification of WO2004 / 001048. Prior to the earliest filing date of WO2004 / 001048, recombinant human arginase can be obtained from the laboratory of Professor Ikemoto Masaki in Japan (Kyoto University; mailing address: 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto-shi, Kyoto 606-8507 Japan). During the test of the drug to be tested, it was prepared with MEM culture solution according to the concentration of the designed dosage group.
[0023] Storage Conditions: Store in a refrigerator at 4°C.
[0024] 1.2 Positive control drug: lamivudine produced by Glaxo Wellcome, UK, batch number: B008923, with a content of 100 mg per tablet. Th...
Embodiment 2
[0044] Embodiment 2: arginase cytotoxicity test
[0045] The experiment was divided into a control group and a drug group with different drug concentrations. Cell digestion, prepared to 200,000 cells per ml, inoculated culture plate, 100 μl per well of 96-well plate, 37°C 5% CO 2 After 24 hours of incubation, the cells were grown into a monolayer before the experiment was performed. Arginase was made into a 40IU / ml solution with culture medium, 2 times diluted 20, 10, 5, 2.5IU / ml and added to a 96-well cell culture plate, a total of 5 dilutions, 3 wells for each concentration, and changed every 4 days Concentration of liquid medicine, with the observation of cell lesions as an index, 8 days or 4 days under the microscope to observe cell lesions, 4 for complete destruction; 3 for 75%; 2 for 50%; 1 for 25%; 0 for no lesion. Calculate the half toxic concentration (TC50) and the maximum non-toxic concentration (TC0) according to the Reed-Muench method.
[0046] TC...
Embodiment 3
[0048] Embodiment 3: Arginase inhibits test to HBeAg, HBsAg
[0049] The test set HBsAg, HBeAg positive control group, negative control group, cell control group and drug groups with different drug concentrations. 2.2.15 Inoculate 200,000 cells per milliliter in a 24-well cell culture plate, 1ml per well, 37°C, 5% CO 2 Cultivate for 24 hours, and dilute the experimental liquid by 2 times below the non-toxic concentration. The 5 dilutions are 20, 10, 5, 2.5, 1.25 IU / ml for arginase, and 800, 400, 200, 100 for lamivudine. , 50μg / ml, 4 wells per concentration, 37°C 5% CO 2 For culture, the original concentration of the drug solution was changed every 4 days, and the culture solution was harvested on the 8th day and stored in a freezer at -20°C. The experiment was repeated in two batches to measure HBsAg and HBeAg respectively. The cpm value per well was measured with a γ-counter.
[0050] Drug effect calculation: Calculate the mean and standard deviation of cpm per concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com