Methods for preventing and treating cancer metastasis and bone loss associated with cancer metastasis
A metastatic cancer and bone technology, applied in chemical instruments and methods, biochemical equipment and methods, pharmaceutical formulations, etc., can solve problems such as bone loss without treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0355] This example demonstrates that highly metastatic breast cancer cell lines express high levels of M-CSF. The M-CSF gene expression level of the highly metastatic cell line MDA231 was compared with the M-CSF gene expression levels of the cell line MCF7 and the cell line ZR751 using a microarray. The result was a 6.9-fold increase in the protein level of M-CSF in MDA231 relative to that in MCF7, and a 5.2-fold increase in the gene expression level in MDA231 compared to ZR751.
Embodiment 2
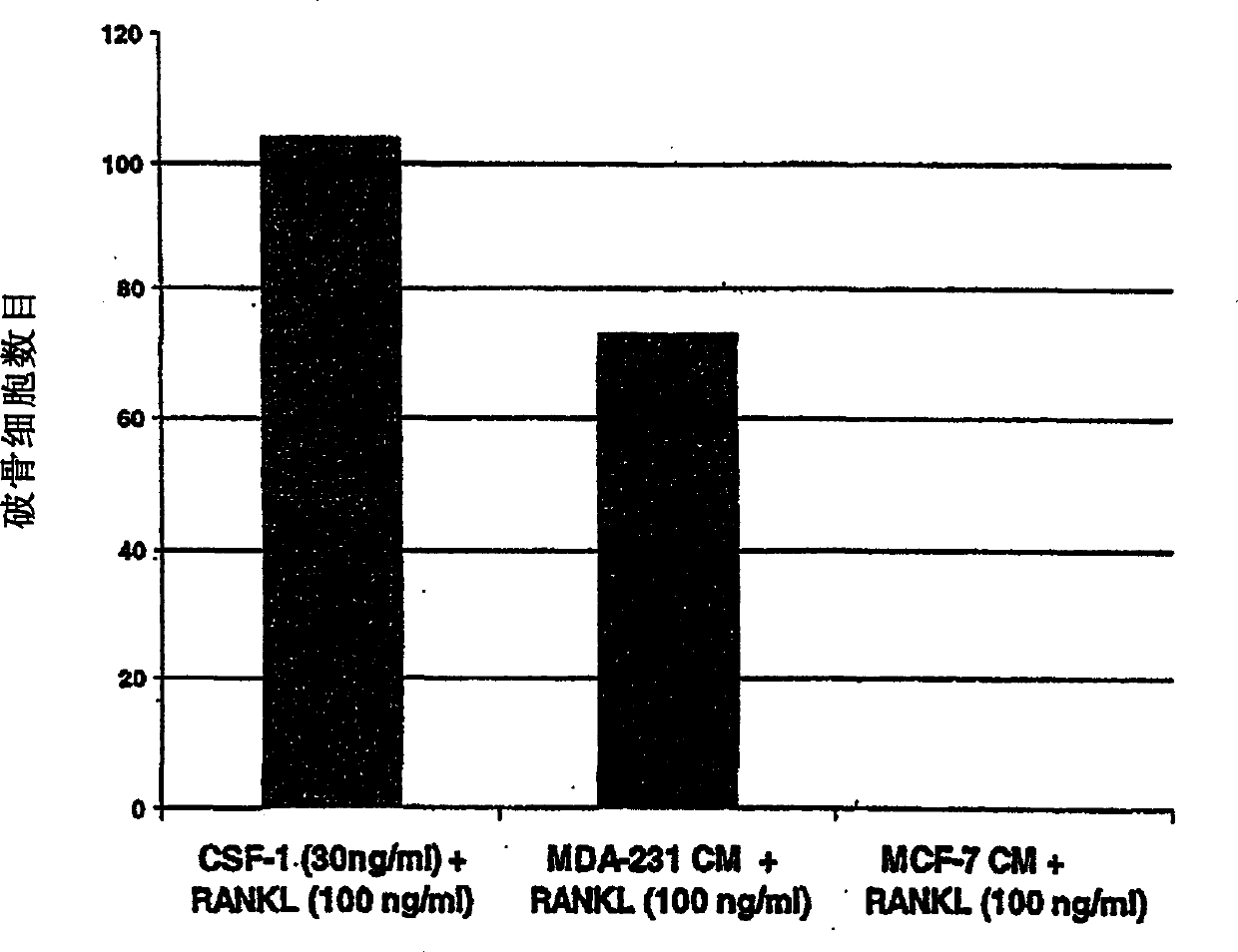
[0357] This example demonstrates that purified M-CSF can be replaced with conditioned medium from the metastatic cell line MDA231 but not with medium from the cell line MCF7 in an in vitro osteoclastogenesis assay (Figure 3 ).
[0358] Production of conditioned medium (CM): MDA231 or MCF7 cells were inoculated into 8 ml of 50% DMEM / 50% HAMs F12 medium containing 1 × ITS (BD Bioscicences, Lexington, KY) at a cell density of 1 × 10 6 / 10 cm Petri dish, which is supplemented medium supplemented with insulin, human transferrin and selenite. at 37°C, 5% CO 2 After 48 hours of incubation under the above conditions, the medium was collected and centrifuged at 1500 RPM for 10 minutes to remove any suspended cells. The supernatant was collected, filtered through a filter membrane with a pore size of 0.2 nm, and used as CM.
[0359] Osteoclast assay: bone marrow CD34 + Cells were seeded in 100 μl of αMEM containing 10% FCS, 1×Pen / Strep and 1× amphotericin B deoxycholate sodium at a ...
Embodiment 3
[0362] This example shows that osteoclasts induced by MDA231 conditioned medium can be neutralized by anti-M-CSF antibody (Figure 4).
[0363] Inoculate bone marrow CD34 as described in Example 2 + cell. The next day, 50 μl of medium was removed from each well. Add 25 μl of 6×antibody 5H4 or αMEM medium to each well, and then add 75 μl of CM or 50% DMEM / 50% HAMs F12 or αMEM medium containing 1×ITS. 100 ng / ml of RANKL was added to all wells, while 30 ng / ml of M-CSF was added to half of the wells. at 37°C, 5% CO 2 cultured for 11 days under the same conditions. During this period, fresh RANKL was added after 6 days. After 11 days, cells were fixed and stained for anti-tartrate acid phosphatase using the leukocyte acid phosphatase kit from Sigma.
[0364] As shown in Figure 4, osteoclasts induced by MDA231-conditioned medium could be neutralized by anti-M-CSF antibody.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap