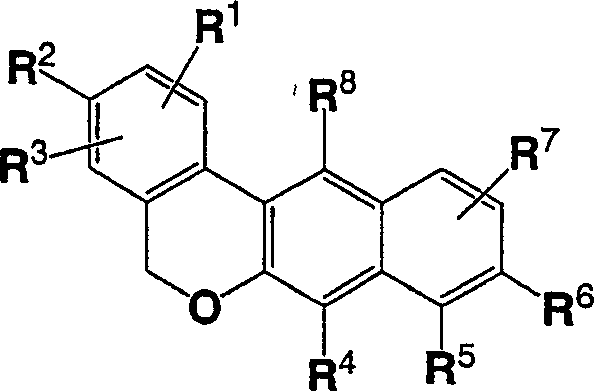
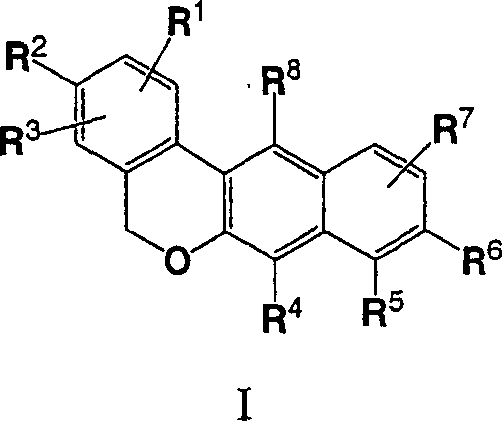
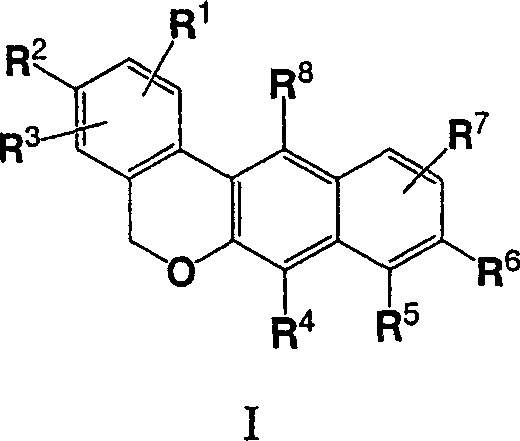
Dibenzo chromene derivatives and their use as ERbeta selective ligands
A technology of diphenyl and chromene, applied in the field of estrogen preparations, can solve problems such as differences in the surrounding environment of co-regulatory proteins
Inactive Publication Date: 2007-04-11
WYETH LLC
View PDF2 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 33
[0079] X = 3-methoxyphenyl Example 34
Embodiment 35
[0081] X=3,4-Difluorophenyl Example 36
Embodiment 38
[0084] X=3,4-dimethylphenyl Example 39
[0085] X=4-cyanophenyl Example 40
[0086] X = 3-fluoro-4-methylphenyl Example 41
[0087] X=3,4-Dimethoxyphenyl Example 42
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
Description
[0001] Cross References to Related Applications [0002] This application claims priority to U.S. Application No. ______, filed February 23, 2005, which claims the benefit of U.S. Application 60 / 547,967, filed February 26, 2004, the disclosures of both applications being incorporated herein by reference in their entirety refer to. technical field [0003] The present invention relates to novel substituted 5H-dibenzo[c,g]chromene derivatives, their use as estrogen preparations and methods for their preparation. Background technique [0004] The pleiotropic effects of estrogen in mammalian tissues have been reported in the literature, and estrogen is now found to affect many organ systems [Mendelsohn and Karas, New England Journal of Medicine 340:1801-1811 (1999), Epperson et al., Psychosomatic Medicine 61: 676-697 (1999), Crandall, Journal of Womens Health & Gender Based Medicine 8: 1155-1166 (1999), Monk and Brodaty, Dementia & Geriatric Cognitive Disorders ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D311/78C07D311/92A61K31/352A61P19/10
Inventor R·E·梅硕R·J·小艾德索
Owner WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com