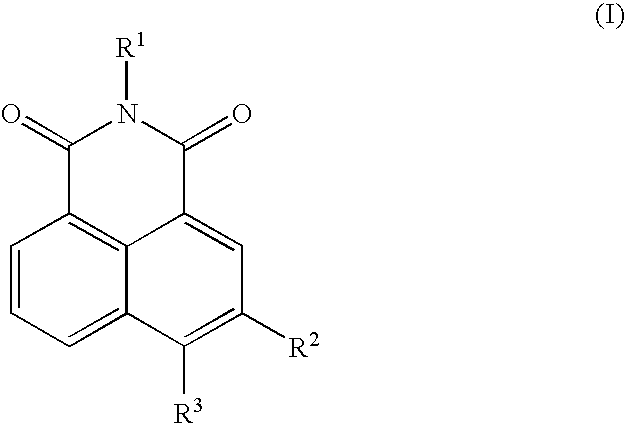
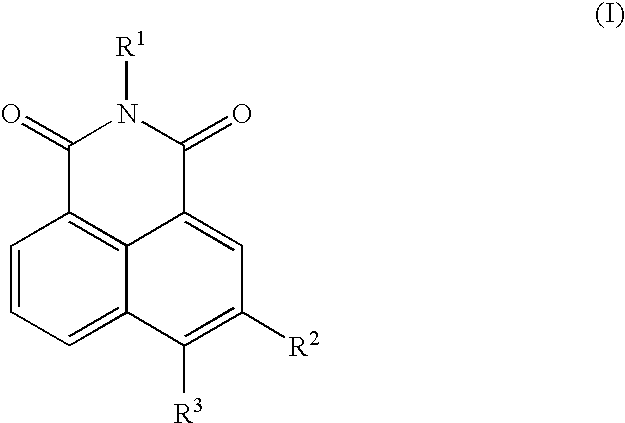
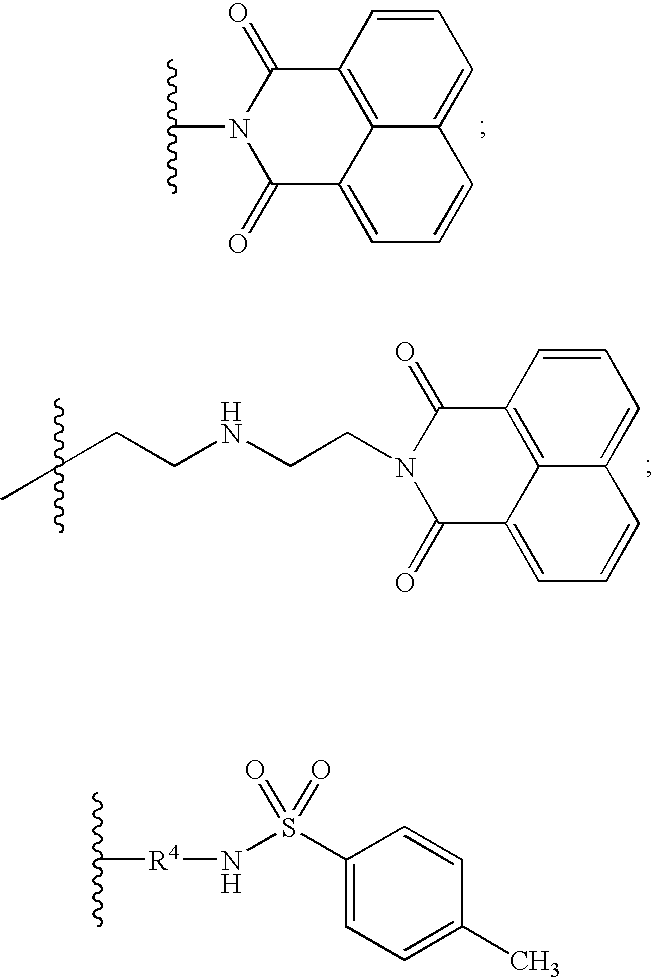
Neurotrophin antagonist compositions
a neurotrophin and composition technology, applied in the field of neurotrophin antagonists, to achieve the effect of inhibiting, or at least reducing, undesirable neurotrophin-mediated activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Binding of [125I]NGF to PC12 Cells in the Presence and Absence of BDNF
[0129]The ability of the compounds of Formula I to antagonize NGF interaction with the p75 and trkA receptors was determined as follows.
(A) Iodination of NGF
[0130]NGF was labelled using the Lactoperoxidase labelling method (Sutter et al., J. Biol. Chem., 1979) and the labelled NGF was separated from radiolabelling agents and free iodide using a PD-10 Sephadex G-25 column.
(B) Cell Culture and Cell Preparation
[0131]PC12 cells were grown in RPMI with 10% heat inactivated donor horse serum and 5% fetal calf serum. Cells were harvested for binding by washing off the media with calcium-magnesium free balanced salt solution (Gey's solution) and incubated in 5 ml Gey's solution at 37° C. for 15 minutes. Cells were pelleted by centrifugation and suspended in Hepes-Krebs Ringer buffer (HKR) (10 mM Hepes pH7.35, containing 125 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4. 1 mg / ml BSA and 1.0 mg / ml glucose) ...
example 2
Inhibition of Neurite Outgrowth
[0133]The ability of N-{5-nitro-1H-benz[de]isoquinoline-1,3(2H)-dione}-2-aminoethanol (Compound A) to inhibit neurite outgrowth was determined using the following assay.
[0134]Eight-day chick embryo dorsal root ganglia (DRG) were freed of meninges and removed aseptically. The DRG were kept at 4° C. at all times. Ganglia from six embryos (40-50 per embryo) were washed in Ca2+- and Mg2+-free Gey's balanced salt solution (Gibco) and exposed to 0.01% trypsin (Worthington) in the same solution for 10 nm u at 37° C. A half-volume of phosphate-buffered Gey's balanced salt solution was added for a further 5 min at 37° C. and the reaction was then terminated with one-third volume of Ham's F12 medium (Gibco) containing 5% fetal calf serum (FCS, Gibco). The ganglia were then triturated using a 5 mL narrow-tip pipette to a single cell suspension. Following filtration through 37-mm nylon mesh (Small Parts Inc., Miami, Fla.) in a Millipore chamber to remove clumps, t...
example 3
Animal Models of Neuropathic Pain
[0137]For pain related to nerve injury, the compound N-{5-nitro-1H-benz[de]isoquinoline-1,3(2H)-dione}-2-aminoethanol (Compound A) was tested in nerve-ligated rats for activity against tactile allodynia, thermal hyperalgesia and in direct production of thermal antinociception. The nerve-ligation model is commonly accepted as representing aspects of neuropathic pain reported by humans. Sham operated rats served as appropriate controls for the neuropathic experiments.
Nerve Ligation Injury:
[0138]Nerve ligation injury was performed according to the method described by Kim and Chung (Pain 50: 355-363, 1992). Rats were anesthetized with halothane and the vertebrae over the L4 to S2 region were exposed. L5 and L6 spinal nerves were exposed, carefully isolated, tightly ligated with 4-0 silk suture distal to the DRG. After ensuring homeostatic stability, the wounds were sutured, and the animals were allowed to recover in the cages. Sham-operated rats were pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com