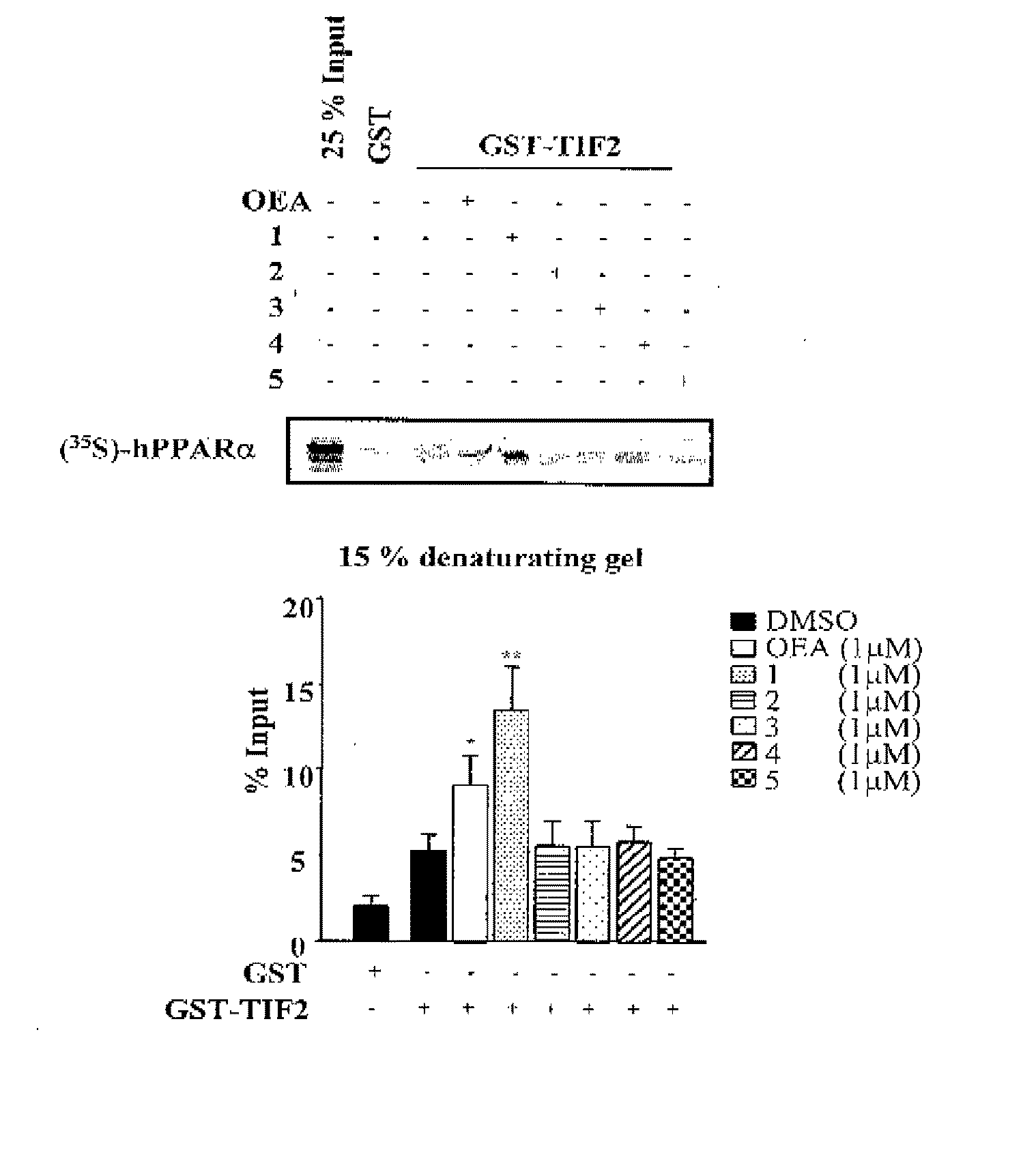
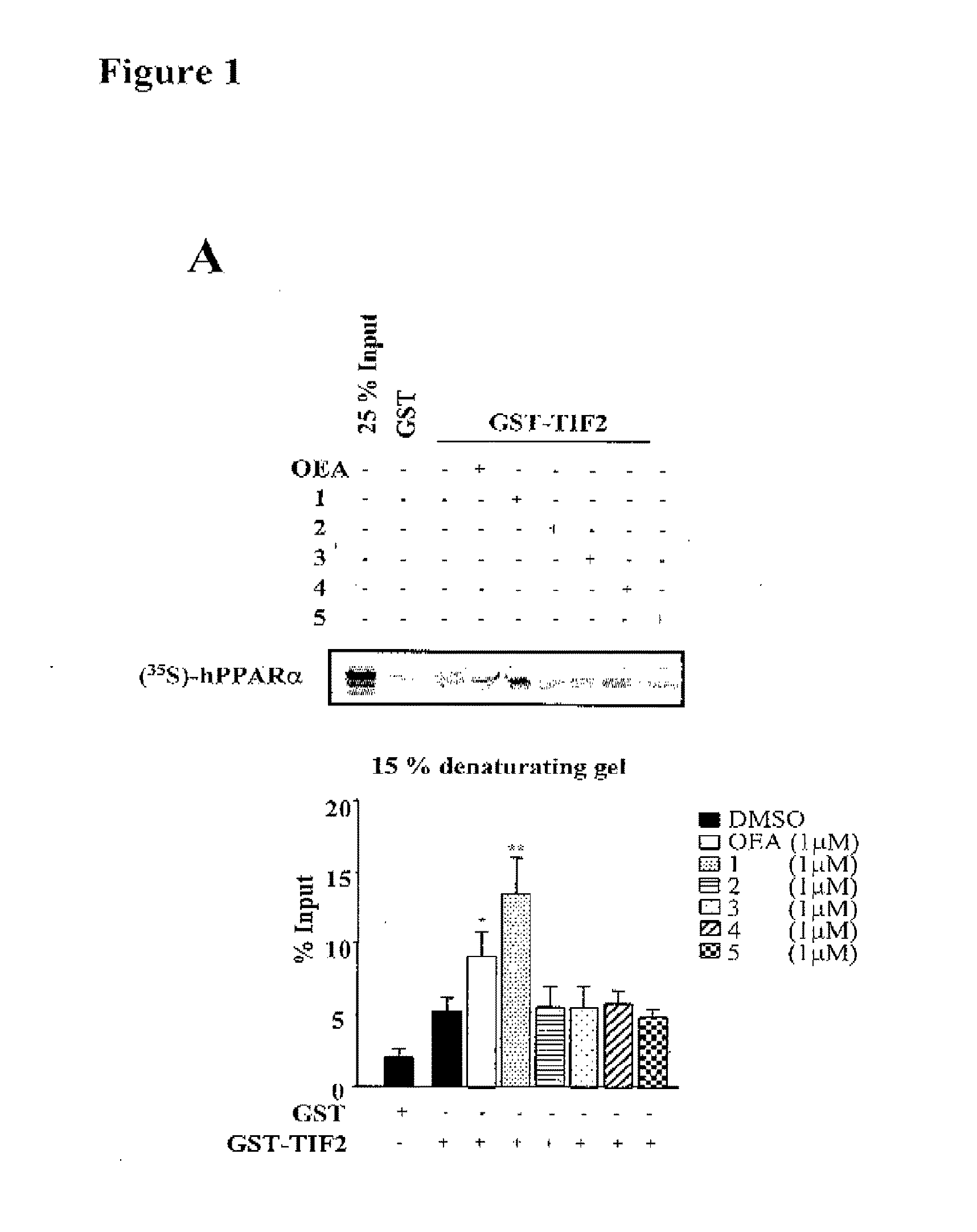
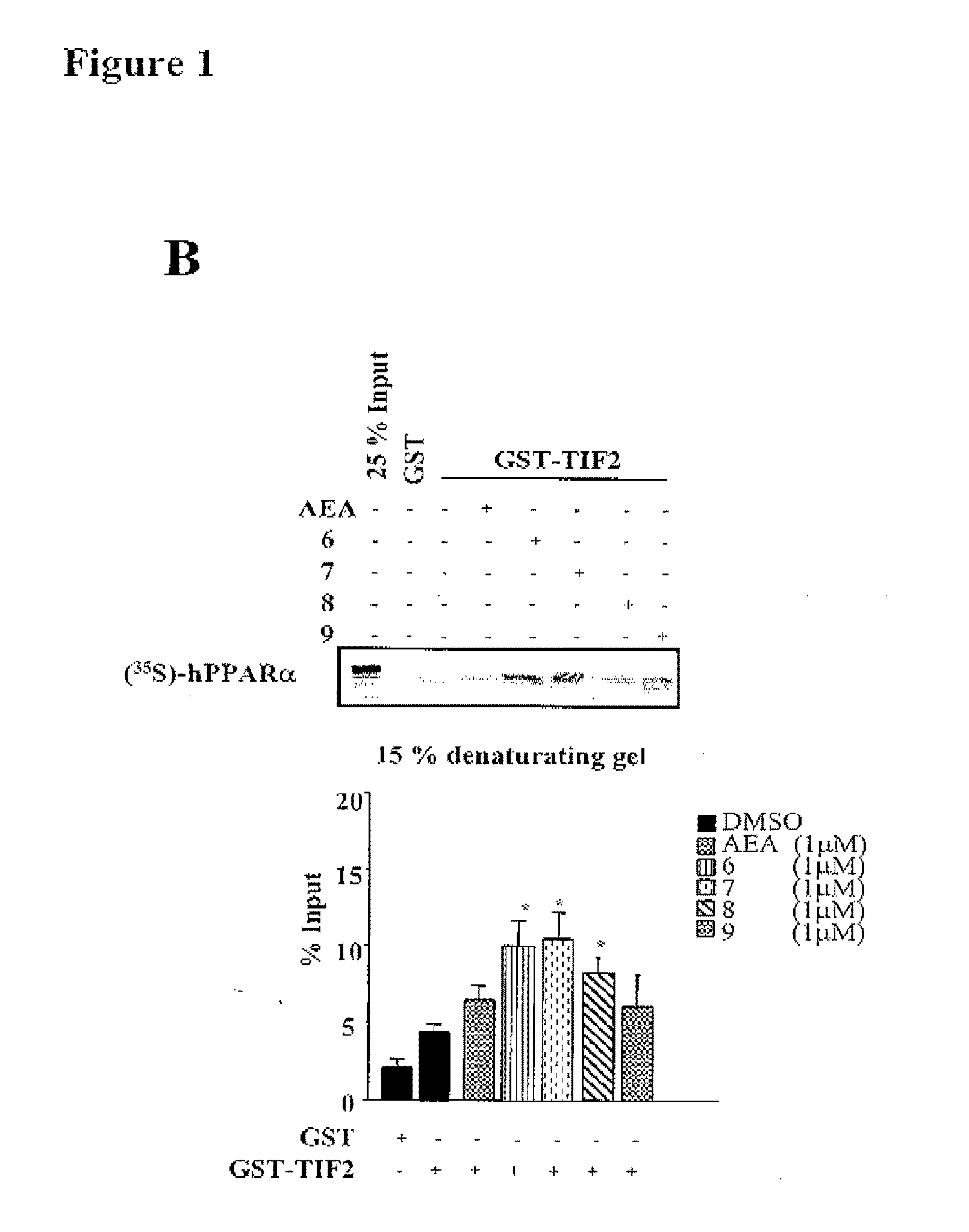
Acyclic Sulfamide Derivatives
a technology of acyclic sulfamide and derivatives, applied in the field of acyclic sulfamide derivatives, can solve problems such as increase in levels, and achieve the effect of reducing cumulative relative weight gain and body weigh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-(cis-9-octadecenyl)sulfamide (1), also known as N-oleylsulfamide
[0133] To a stirred solution of 0.20 g of sulfamide (2.1 mmol) in 20 ml of H2O under reflux it is added dropwise 0.1 ml (2.1 mmol) of N-oleylamine in 10 ml of EtOH for 30 minutes. The reaction mixture is stirred and refluxed for 6 hours. Then the solvent is evaporated and the white solid is purified in a chromatography column, using CH2Cl2:MeOH / NH3 9.4:0.6 as eluyent; 0.52 g of white solid are obtained. Yield 72%; mp=68-70 C;
[0134]1H NMR (300 MHz, CDCl3): 5.32 (m, 2H); 4.59 (br s, 2H); 4.38 (br s, 1H); 3.09 (q, J=7.0 Hz, 2H); 1.97 (m, 4H); 1.55 (m, 2H); 1.24 (m, 22H); 0.85 (t, J=6.7 Hz, 3H).
[0135]13C NMR (75 MHz, CDCl3): 129.9; 129.7; 43.6; 31.8; 29.7-26.6; 27.1; 27.2; 22.6; 14.1.
[0136] MS (ES+) [M+H]+347 (100%). Anal. (C18H38N2SO2, 346.57): C, H, N, S.
example 2
Preparation of N-octadecylsulfamide (2)
[0137] 0.270 g, (1.0 mmol) of N-octadecylamine and 0.100 g (1.0 mmol) of sulfamide in 50 ml of THF (50 mL) are stirred and refluxed for 51 h. The solvent is evaporated and the crude is purified in a chromatography column, using CH2Cl2:MeOH / NH3 9:1 as eluyent. 0.06 g of white solid are obtained. Yield 18%; mp=105-107° C.;
[0138]1H NMR (400 MHz, DMSO-d6): 6.39 (br s, 2H); 6.33 (br s, 1H); 2.82 (m, 2H); 1.42 (m, 2H); 1.21 (bm, 30H); 0.83 (t, J=6.8 Hz, 3H).
[0139]13C NMR (100 MHz, DMSO-d6): 42.7; 31.4; 29.1; 26.5; 22.2; 14.1;
[0140] MS (ES+) [M+H]+349 (100%). Anal. (C18H40N2SO2, 348.64): C, H, N, S.
example 3
Preparation of N-hexadecylsulfamide (3)
[0141] The title compound was prepared following the procedure previously described in Example 1, using 0.5 g (2.0 mmol) of N-hexadecylamine and 0.195 g (2.0 mmol) of sulfamide as starting reagent. 0.087 g of a white solid were obtained. Yield 16%; m.p. 102-104° C.
[0142]1H-NMR (500 MHz, DMSO-d6) δ: 2.83 (c, 2H, |J|=6.8 Hz CH2NHSO2); 1.43 (1, 2H, |J|=6.8 Hz, CH2—CH2NHSO2); 1.23 (s, 20H, —CH2—); 0.85 (t, 3H, |J|=6.8 Hz, CH2—CH3).
[0143]13C-NMR (125 MHz, DMSO-d6) δ: 42.5 (CH2NH); 31.3 (CH2—CH2NH); 29.0-28.7 (—CH2—); 26.4 (CH2—CH2—CH2NH); 22.1 (CH2—CH3); 13.9 (CH2—CH3).
[0144] Anal (C16H36N2SO2) % theor. (% found): C, 59.95 (59.68); H, 11.32 (11.04); N, 8.74 (8.65); S, 10.00 (9.91).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com