Methods for determining the prognosis for patients with a prostate neoplastic condition
a prostate neoplastic and prognosis technology, applied in the field of prostate neoplastic patients prognosis determination, can solve the problems of inability to accurately predict the course of disease for all prostate cancer patients, dilemma in treatment decisions, urinary urgency, etc., and achieve the effect of determining the prognosis for survival, increasing the level of xiap, and increasing the survival rate of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example i
Expression of Inhibitor of Apoptosis (IAP) Polypeptides in Prostate Cancer
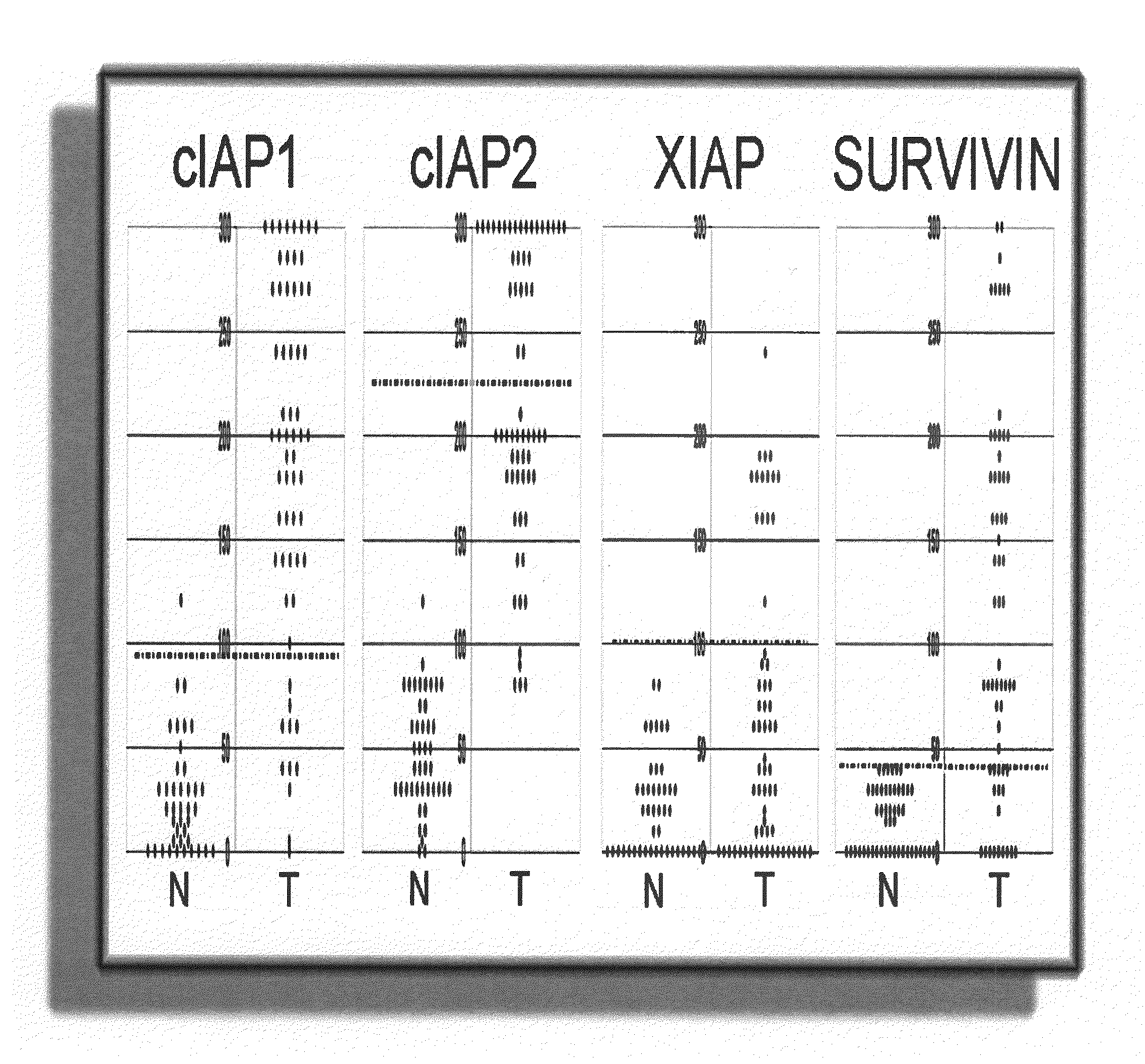
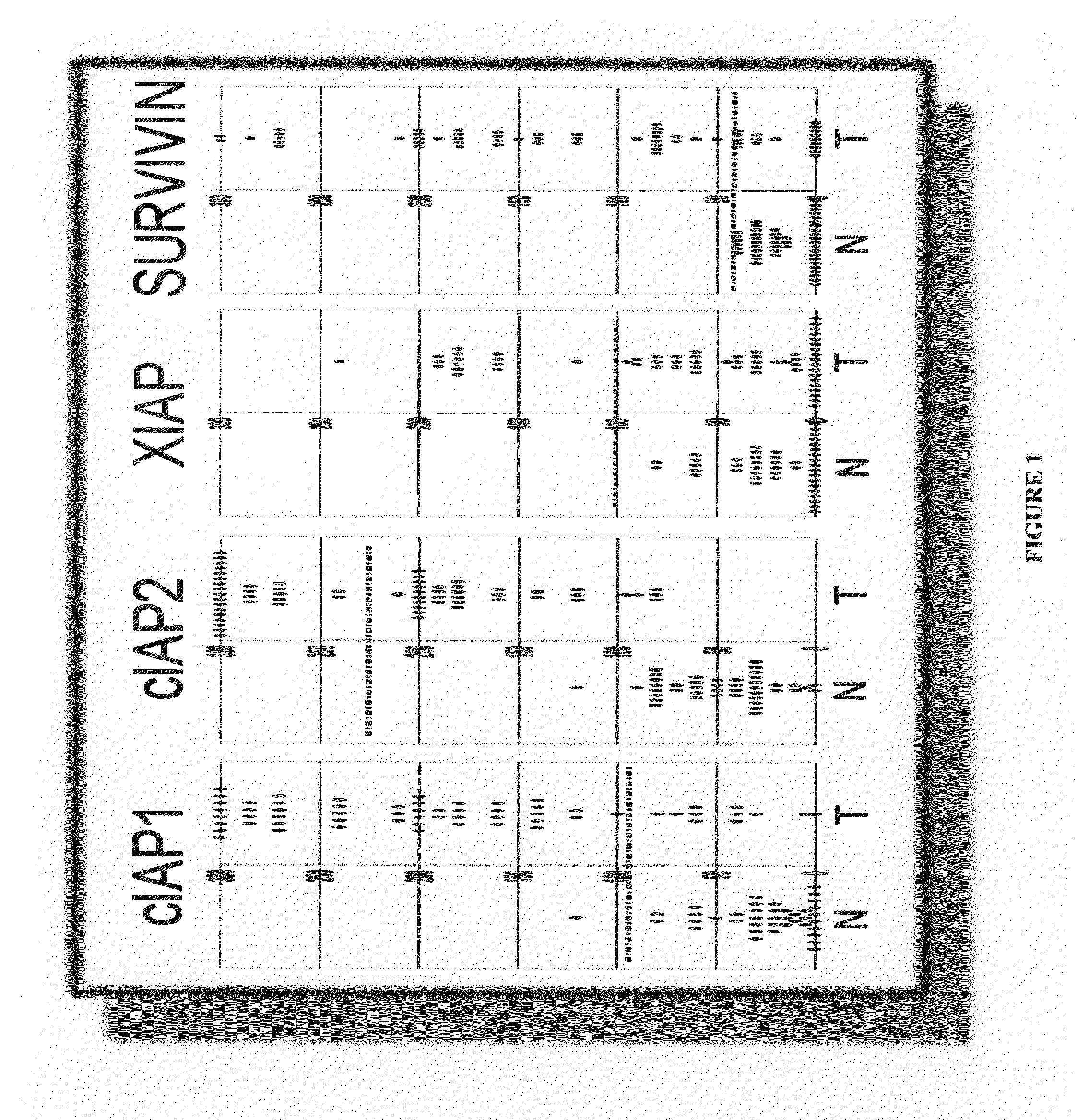
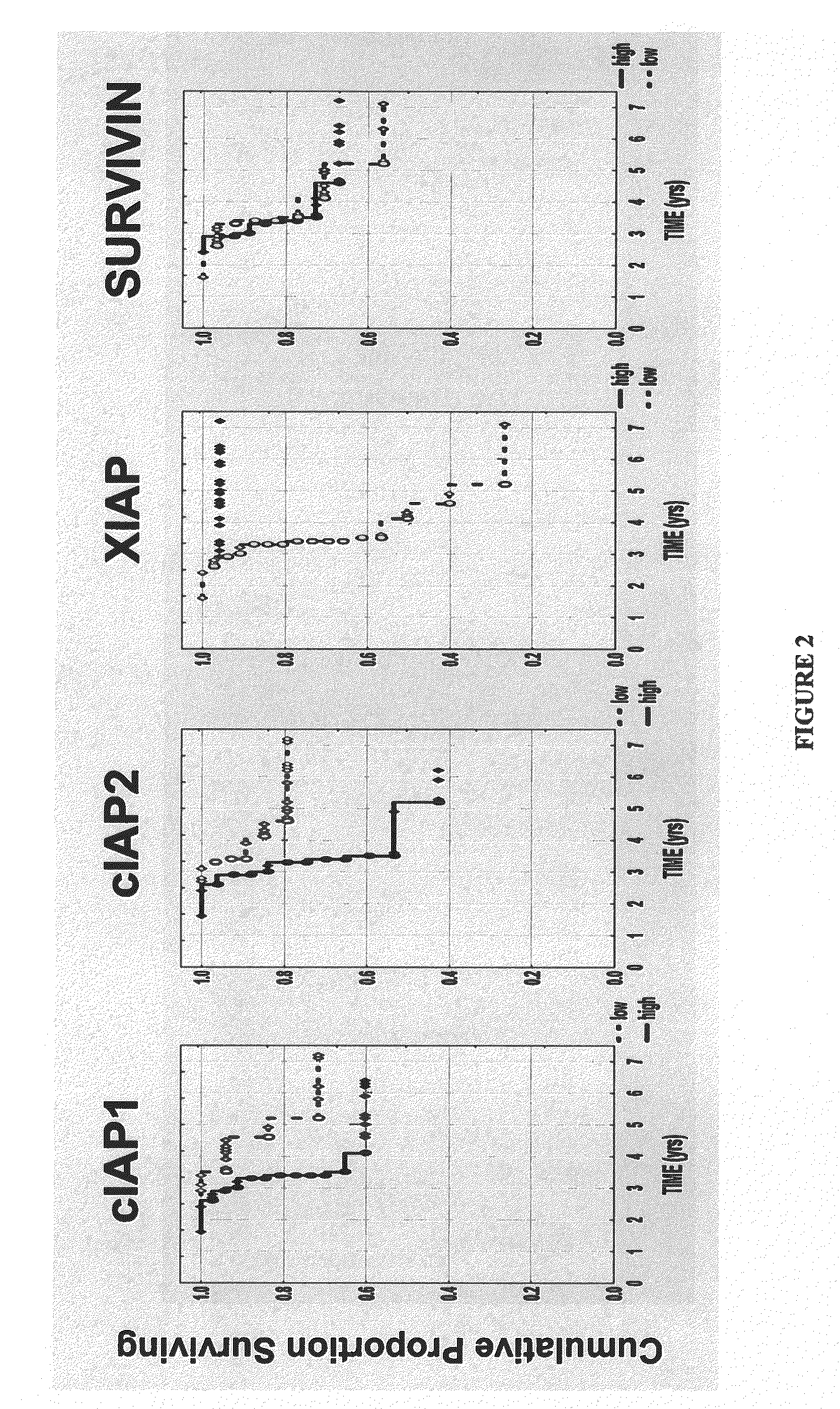
[0163]This example shows a method for identifying biomarkers that correlate to prostate cancer patient survival. A study was performed for IAP-family members on needle biopsy specimens for a cohort of 62 men with stage II peripheral zone prostate carcinomas treated by external beam irradiation. Stage II disease is also known as stage B (B0, B1, B2 combined) disease. Cancer progression during a median follow-up of 66 months was defined as biochemical recurrence (3 consecutive rises in prostate specific antigen (PSA) concentration). Of 16 / 62 (26%) patients classified as alive with disease (AWD) with regard to biochemical failure, 15 patients developed metastatic disease documented by bone scans.
[0164]Briefly, 62 adenocarcinoma specimens and 40-48 case-matched samples containing normal prostatic epithelium were immunostained and evaluated for the presence of cIAP1, cIAP2, XIAP, and Survivin polypeptides. The mean...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| PSA | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap