Nucleic Acids and Libraries
a technology applied in the field of nucleic acids and libraries, can solve the problems of generating a substantial number of false positives, difficult target identification task, and prediction likely to be inherently biased
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Nucleic Acids
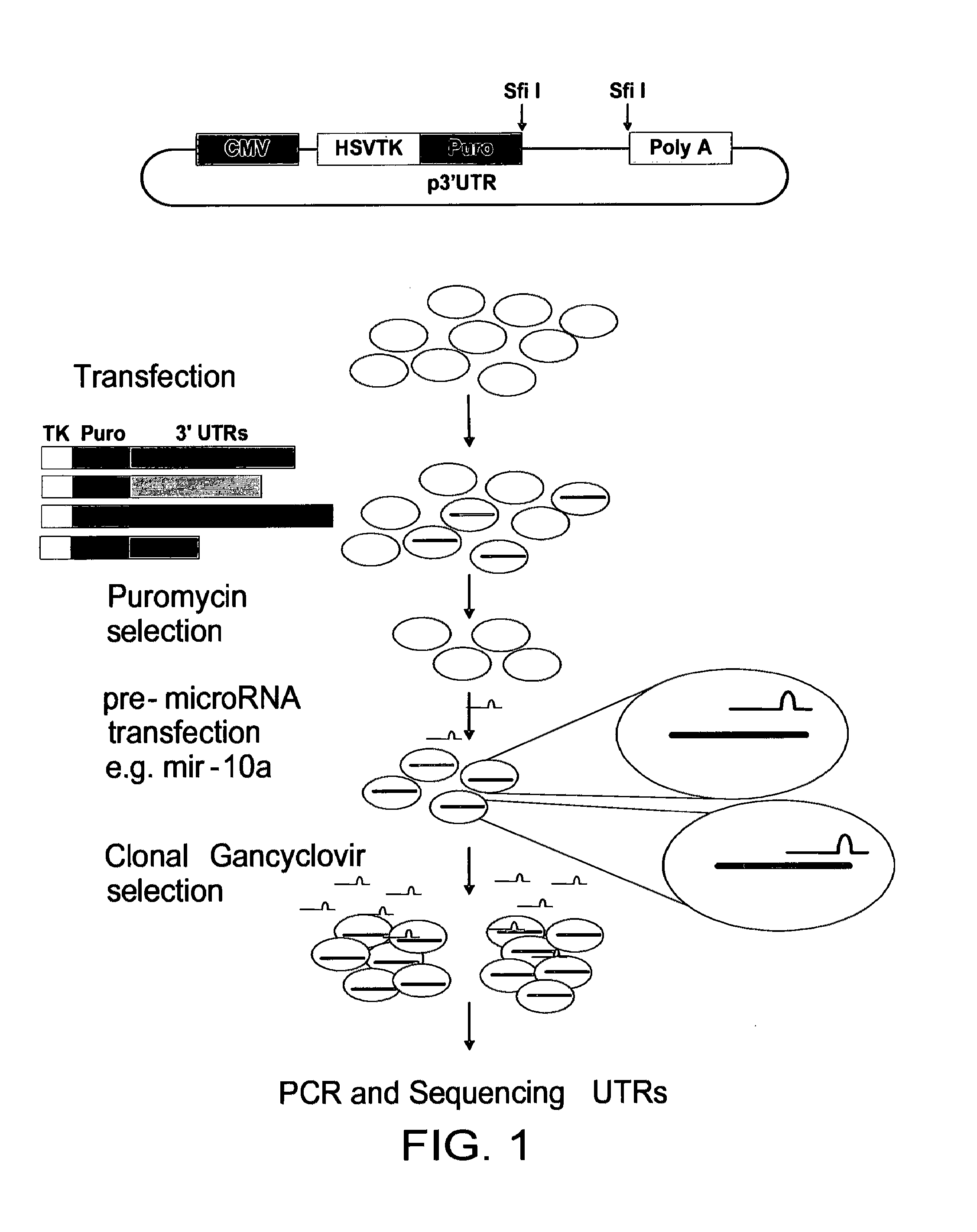
[0056]A nucleic acid is constructed comprising the following contiguous elements arranged in the 5 prime to 3 prime direction; a promoter; a selectable marker; a cloning site for receipt of a nucleic acid segment, said segment comprising a candidate miRNA target sequence; and a poly adenylation signal.
[0057]The elements are arranged such that a transcript directed by said promoter comprises said selectable marker, said candidate miRNA target sequence, and said poly adenylation signal in that order.
example 2
Dual Selectable Markers
[0058]As explained herein, the selectable marker is a key part of the present invention. In certain embodiments, the selectable marker may advantageously comprise more than one activity. This example demonstrates the production of selectable markers with more than one activity. In this example, this is accomplished by fusion of the ORFs for two different individual selectable markers into a single nucleic acid segment. This advantageously results in the production of a single polypeptide comprising two different polypeptide domains, each having its specific (selectable) activity.
[0059]In this example, the two individual markers used are HSVTK and PURO. These are fused to form a TK / PURO dual selectable marker.
[0060]The open reading frames of HSVTK and PURO are studied. A suitable fusion point is selected with consideration to the nature of the polypeptide products in order to maximise the chances of their activity being retained in the fused product. At this st...
example 3
HSVTK / PURO Dual Selectable Marker
[0063]In this example, the two selectable markers are fused to produce a single translation product comprising both activities / polypeptides.
[0064]In this example, the two individual markers used are HSVTK and PURO. These are fused to form a TK / PURO dual selectable marker.
[0065]The open reading frames of HSVTK and PURO are studied. The markers are then fused as described in example 2.
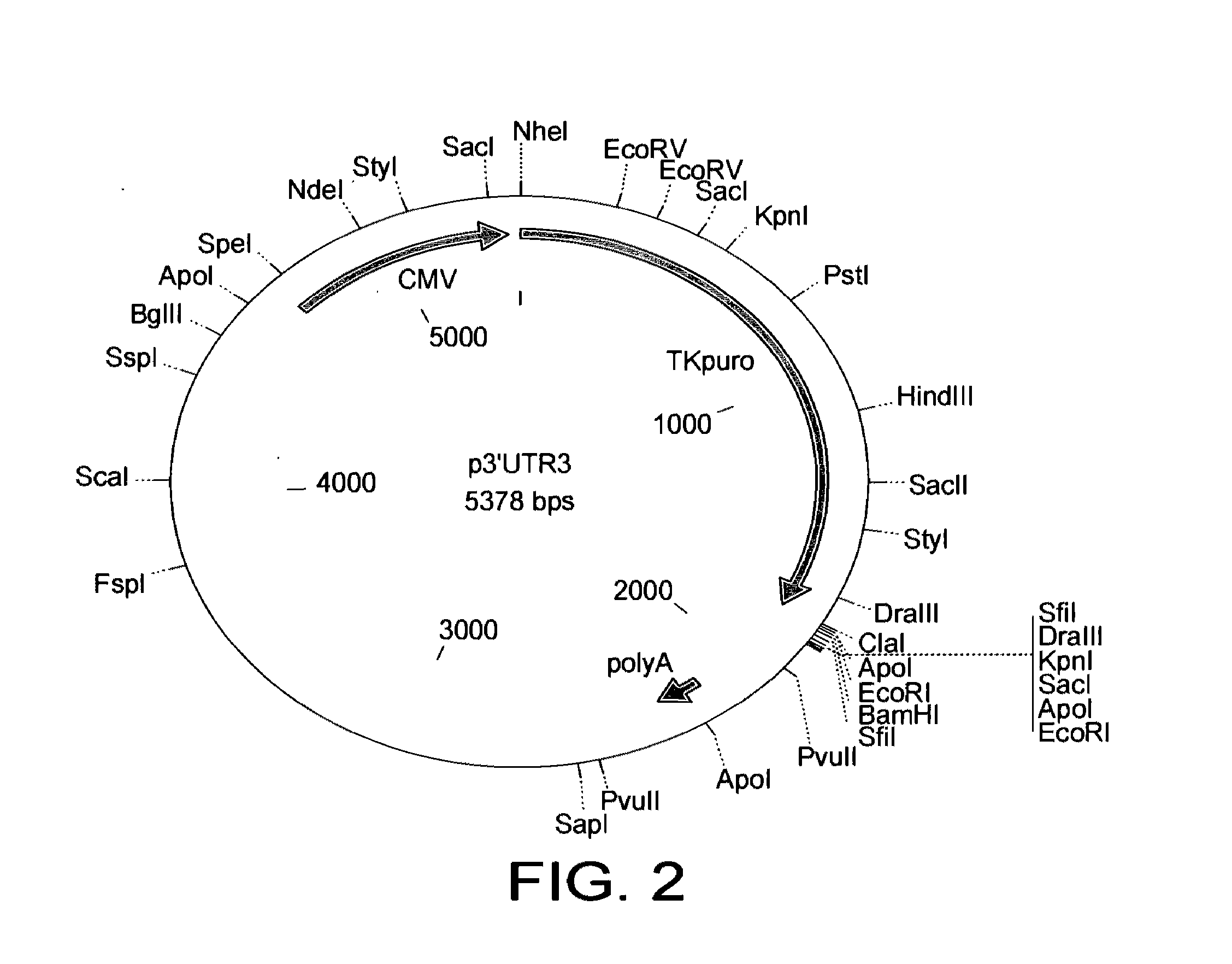
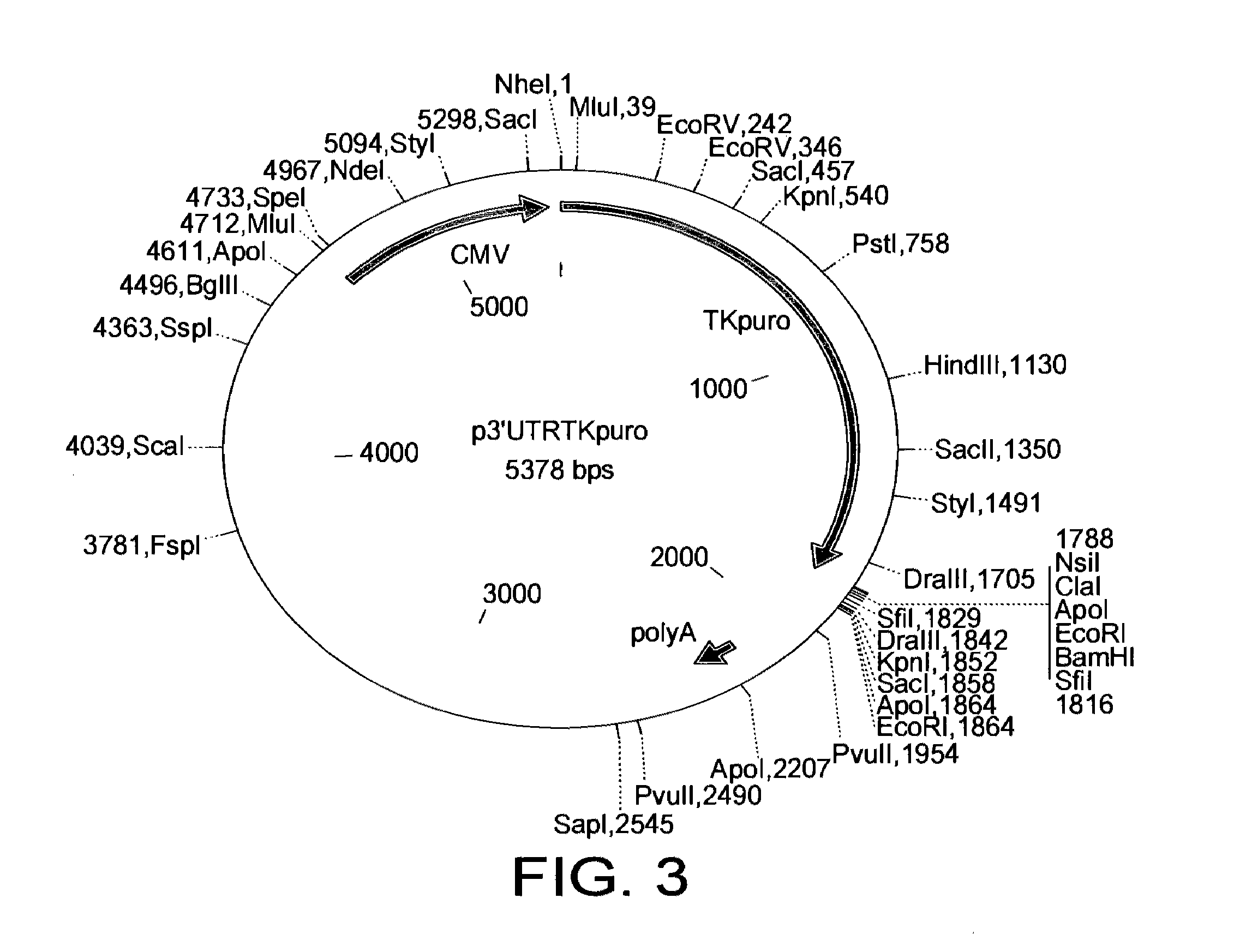
[0066]The resulting selectable marker is shown in SEQ ID NO: 1. This is a dual selectable marker. This is a TK-PURO fusion according to the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| cell adhesion | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com