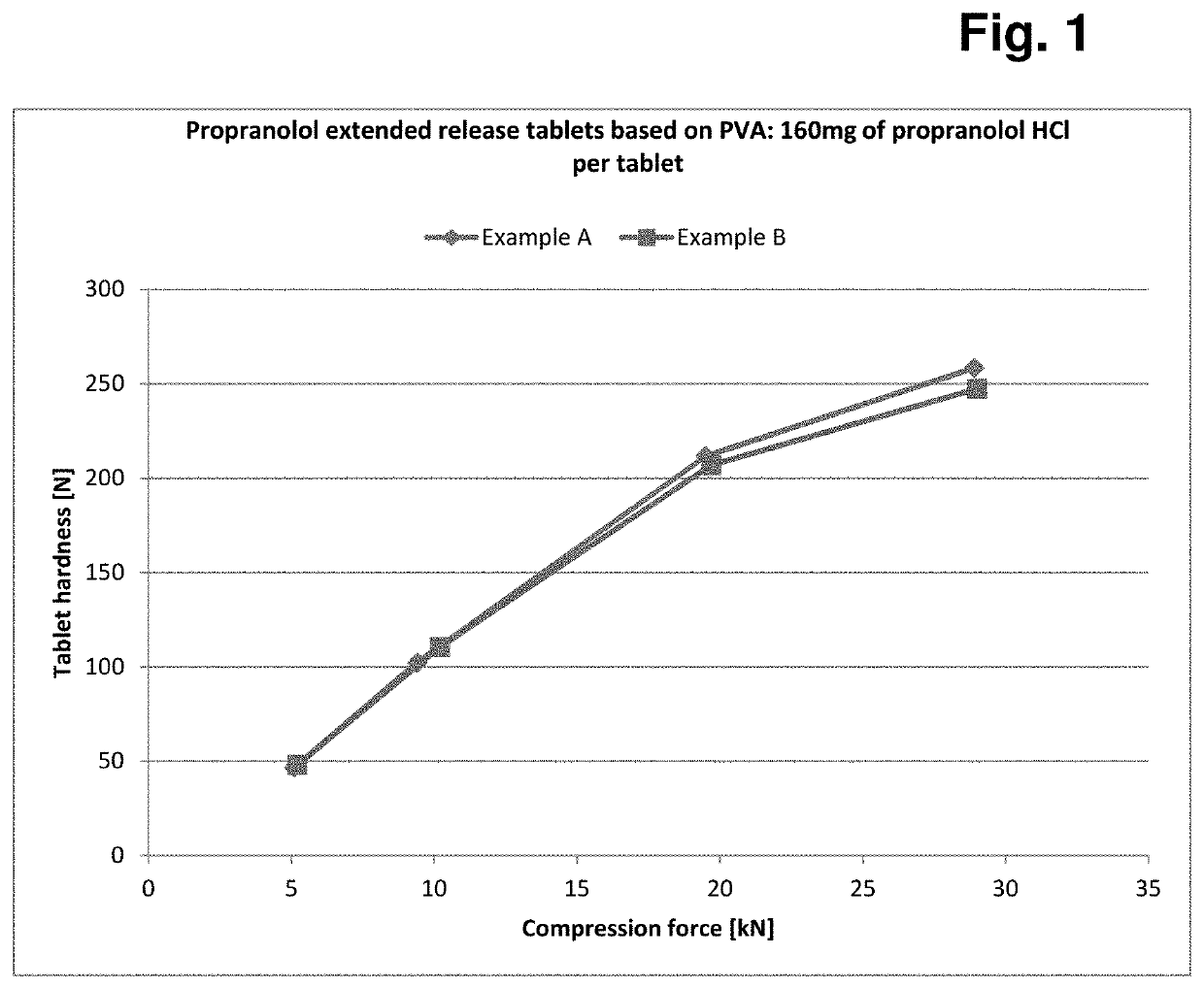
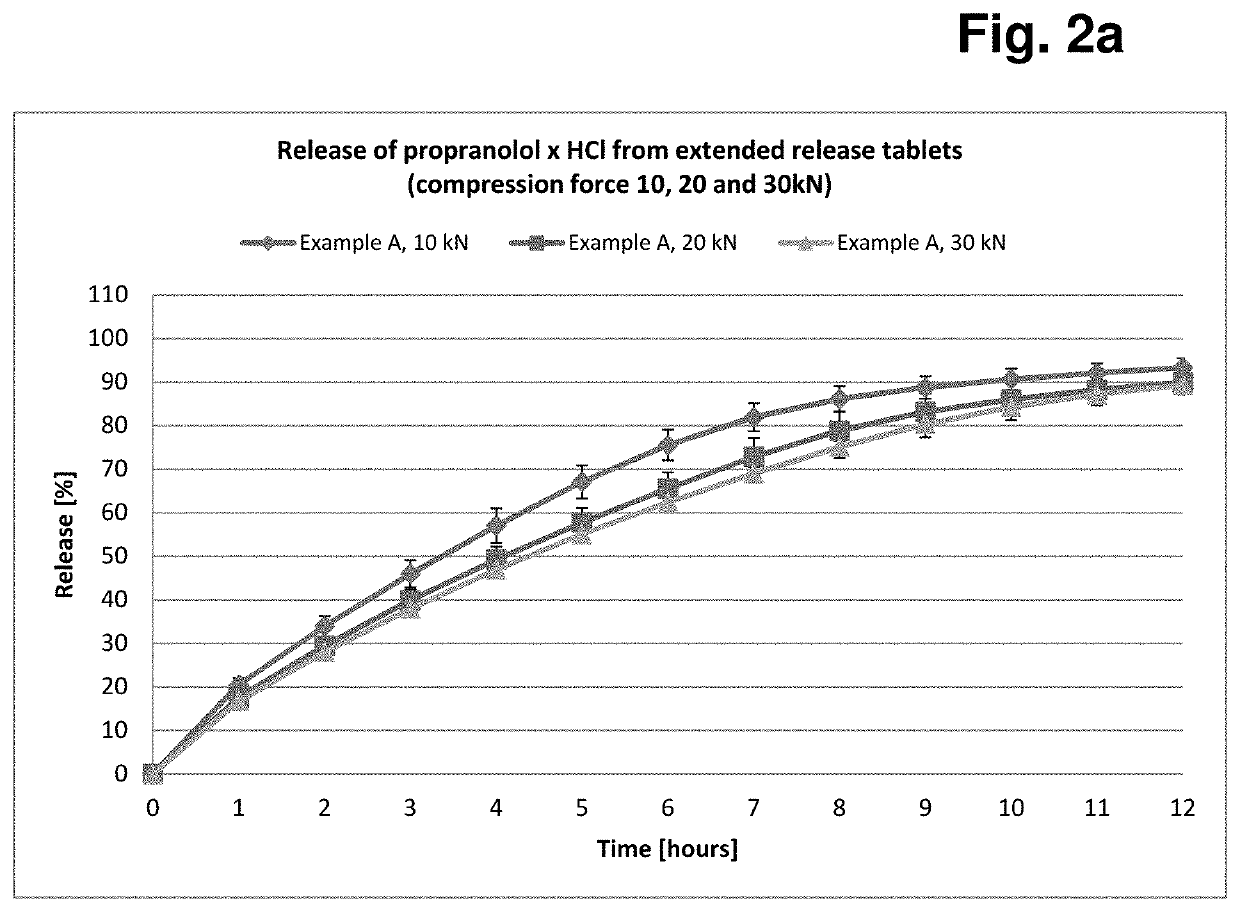
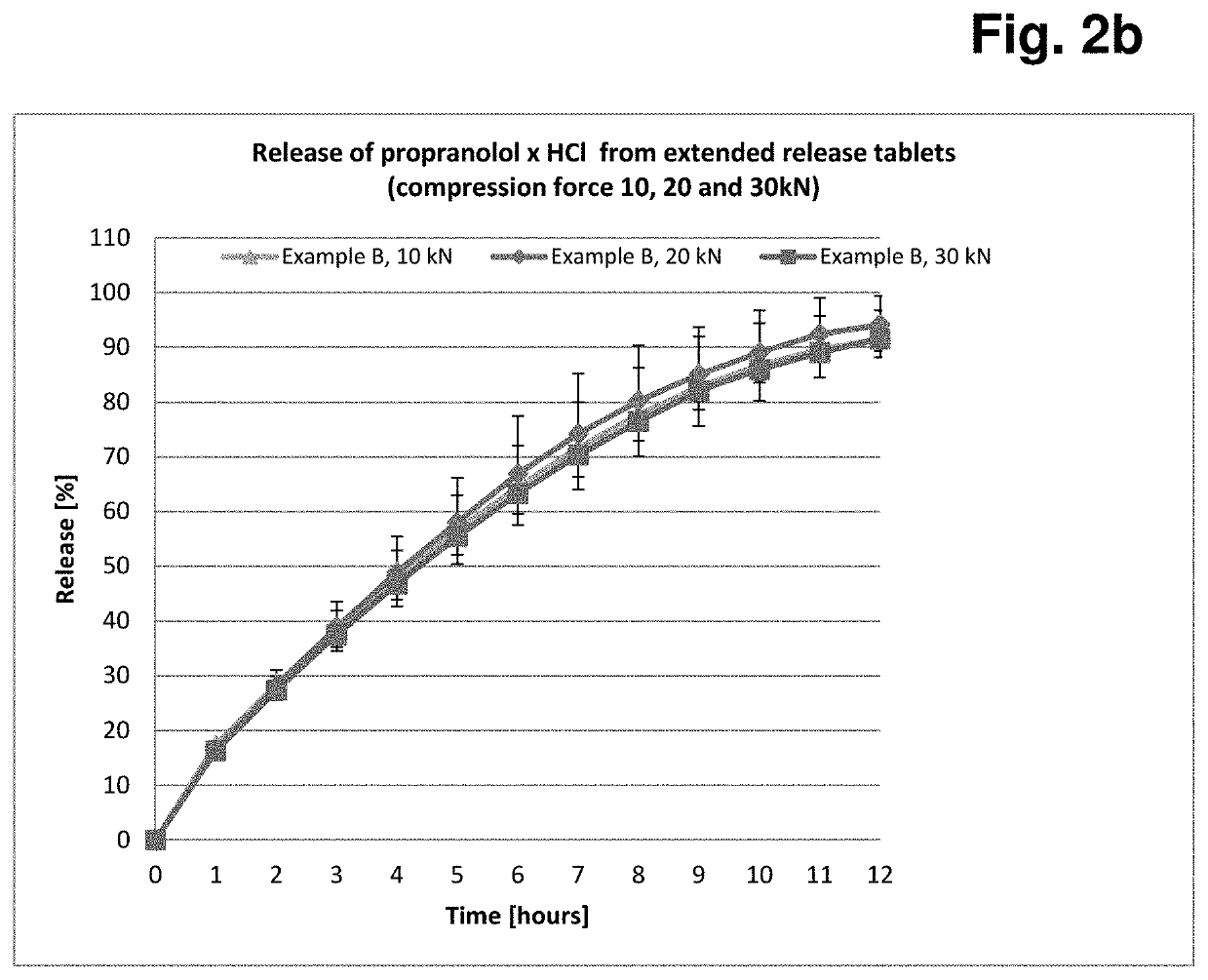
Formulation having controlled, delayed release of active ingredient
a technology of active ingredients and formulations, applied in the direction of heterocyclic compound active ingredients, drug compositions, cardiovascular disorders, etc., can solve the problems of short elimination half-life of 2-6 hours, and limited bioavailability of about 25-30%, so as to avoid undesired dose dumping of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0086]Instruments and methods for the characterisation of the material properties[0087]1. Bulk density: in accordance with DIN EN ISO 60: 1999 (German version)[0088]quoted in “g / ml”[0089]2. Tapped density: in accordance with DIN EN ISO 787-11:1995 (German version)[0090]quoted in “g / ml”[0091]3. Angle of repose: in accordance with DIN ISO 4324: 1983 (German version)[0092]quoted in “degrees”[0093]4. Surface area determined by the BET method: evaluation and procedure in accordance with the literature “BET Surface Area by Nitrogen Absorption” by S. Brunauer et al. (Journal of American Chemical Society, 60, 9, 1983). Instrument: ASAP 2420 Micromeritics Instrument Corporation (USA); nitrogen; sample weight: about 3.0000 g; heating: 50° C. (5 h); heating rate 3 K / min; arithmetic mean from three determinations quoted[0094]5. Particle size determination by laser diffraction with dry dispersal: Mastersizer 2000 with Scirocco 2000 dispersion unit (Malvern Instruments Ltd., UK), determinations a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size Dv50 | aaaaa | aaaaa |
| particle size Dv50 | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com