Inhibitors of Akt activity
A kind of compound, the technology of dihydrogen, be used in the compound field containing heterocyclic triazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
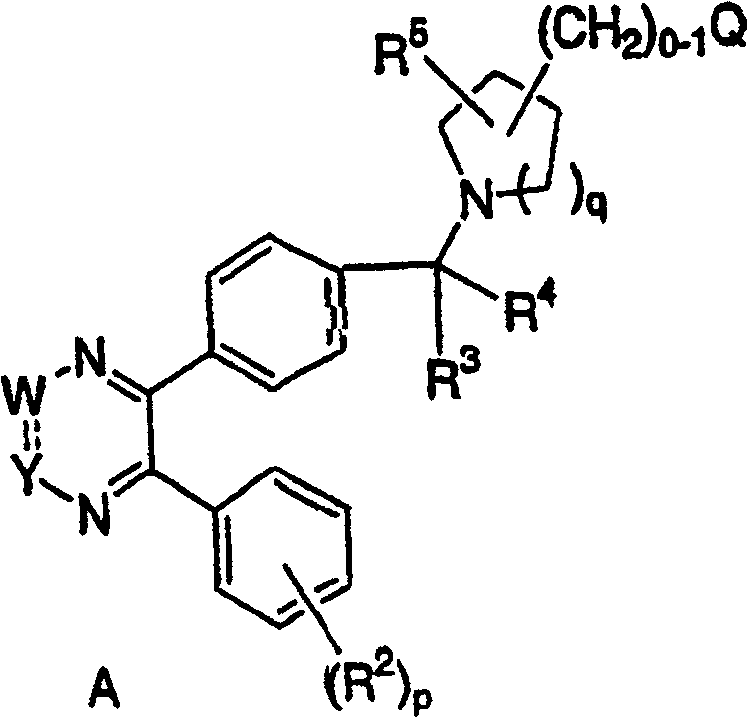
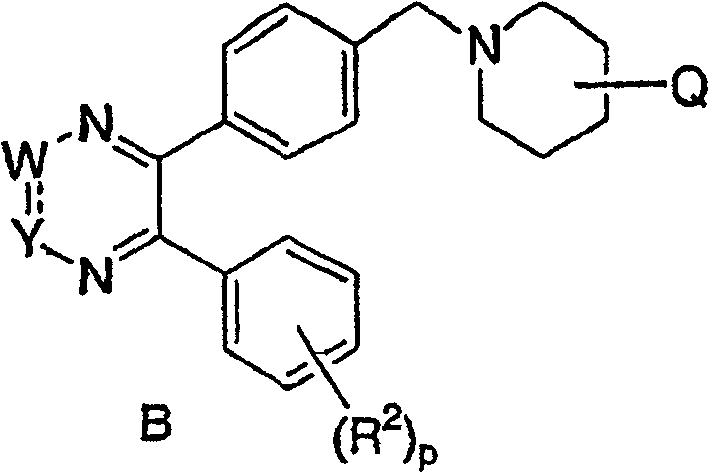
[0035] The third embodiment of the present invention is a compound represented by formula B, or a pharmaceutically acceptable salt or stereoisomer thereof, wherein:
[0036] Q selected from: optional by 1-3 R z substituted-NR 6 R 7 , phenyl and heterocyclyl;
[0037] R a For: (C 1 -C 6 ) Alkyl, (C 3 -C 6 ) cycloalkyl, aryl or heterocyclyl; and
[0038] R b For: H, (C 1 -C 6 ) Alkyl, aryl, heterocyclyl, (C 3 -C 6 ) cycloalkyl, (C = O) OC 1 -C 6 Alkyl, (C=O)C 1 -C 6 Alkyl or S(O) 2 R a ; All other substituents and variables are as defined in the second embodiment.
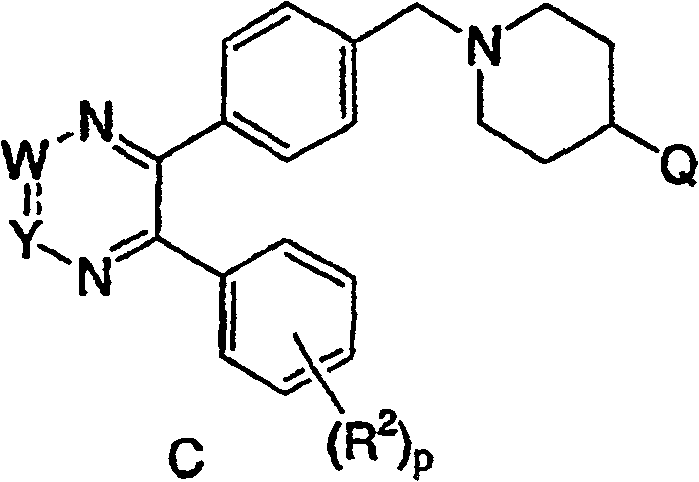
[0039] The fourth embodiment of the present invention is a compound represented by the following formula C, or a pharmaceutically acceptable salt or stereoisomer thereof:
[0040]
[0041] in:
[0042] Q is a heterocyclic group, and the heterocyclic group is optionally replaced by 1-3 R z replace;
[0043] R 2 Independently selected from: 1) C 1 -C 6 Alkyl, 2) aryl, 3) heterocyclyl, 4) CO...
Embodiment 1
[0277] Cloning of human Akt isoforms and ΔPH-Akt1
[0278] The pS2neo vector (deposited at ATCC on April 3, 2001 with the accession number ATCCPTA-3253) was prepared as follows: cut the pRmHA3 vector with BglII (according to the introduction in Nucl.Acid Res.16:1043-1061 (1988) method), and a 2734 bp fragment was isolated. The pUChsneo vector (prepared according to the method described in EMBO J.4: 167-171 (1985)) was also cut with BglII, and a 4029 bp fragment was isolated. The two isolated fragments ligated to generate the vector, called pS2neo-1. This plasmid contains a polylinker between the metallothionein promoter and the alcohol dehydrogenase polyA addition site. It also has a neo resistance driven by a heat shock promoter Gene. The pS2neo-1 vector was cut with Psp5II and BsiWI. Two complementary oligonucleotides were synthesized and then annealed (CTGCGGCCGC (SEQ.ID.NO.: 1) and GTACGCGGCCGCAG (SEQ.ID.NO.2)). Cut pS2neo-1 was ligated with annealed oligonucleotides to...
Embodiment 2
[0301] Expression of human Akt isoforms and ΔPH-Akt1
[0302] Using the calcium phosphate method, the DNA containing the cloned Akt1, Akt2, Akt3 and ΔPH-Akt1 genes in the pS2neo expression vector was purified, and transfected into Drosophila (Drosophila) S2 cells (ATCC). Select antibiotics (G418, 500 μg / ml) Resistant cell aggregates. Dilute the cells to a volume of 1.0L (approximately 7.0×10 6 / ml), adding biotin and CuSO 4 , so that the final concentrations were 50 μM and 50 mM respectively. The cells were grown at 27°C for 72 hours and harvested by centrifugation. The cell pellets were stored at -70°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com