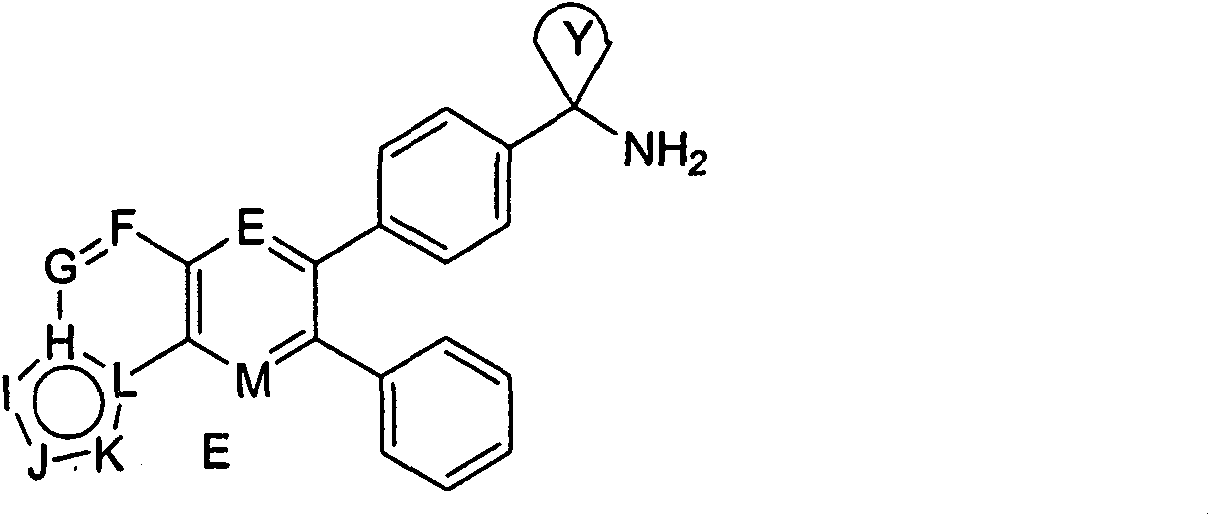
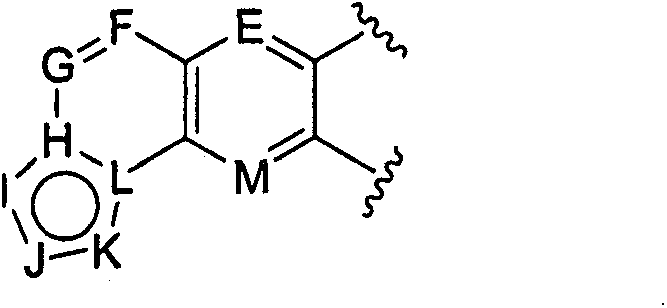
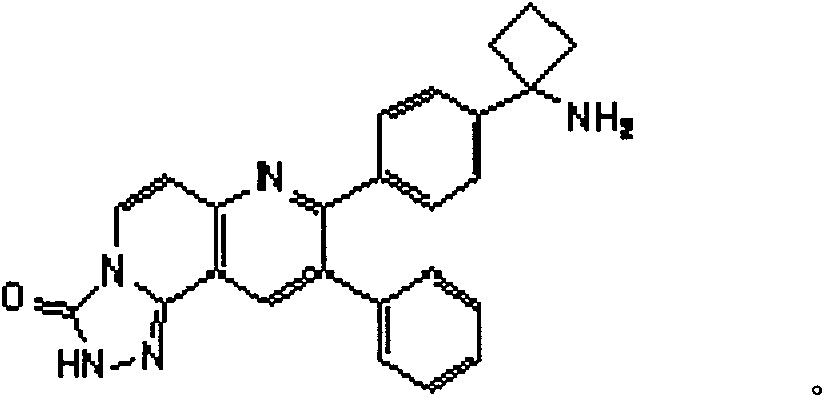
Inhibitors of AKT activity
A technology of phenyl and naphthalene, which is applied in the direction of organic active ingredients, non-central analgesics, anti-inflammatory agents, etc., can solve the problem of no specific PDK1 inhibitor yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0746] Cloning of human Akt isoforms and ΔPH-Akt1
[0747] The pS2neo vector (deposited at ATCC on April 3, 2001 with the accession number ATCCPTA-3253) was prepared as follows: cut the pRmHA3 vector with BglII (according to the method introduced in Nucl.AcidRes.16:1043-1061 (1988) Preparation), a 2734bp fragment was isolated. The pUChsneo vector (prepared according to the method described in EMBO J. 4: 167-171 (1985)) was also cut with BglII, and a 4029 bp fragment was isolated. The two isolated fragments were ligated to generate the vector, called pS2neo-1. This plasmid contains a polylinker between the metallothionein promoter and the alcohol dehydrogenase polyA addition site. It also has a neo resistance gene driven by a heat shock promoter. The pS2neo-1 vector was cut with Psp5II and BsiWI. Two complementary oligonucleotides were synthesized and then annealed (CTGCGGCCGC (SEQ. ID. NO.: 1) and GTACGCGGCCGCAG (SEQ. ID. NO.: 2)). The cut pS2neo-1 was ligated with the ...
Embodiment 2
[0763] Expression of human Akt isoforms and ΔPH-Akt1
[0764] Using the calcium phosphate method, the DNA containing the cloned Akt1, Akt2, Akt3 and ΔPH-Akt1 genes in the pS2neo expression vector was purified and transfected into Drosophila S2 cells (ATCC). Antibiotic (G418, 500 μg / ml) resistant cell aggregates were selected. Expand the cells to a volume of 1.0L (approximately 7.0×10 6 / ml), adding biotin and CuSO 4 , so that the final concentrations were 50μM and 50mM. Cells were grown for 72 hours at 27°C and harvested by centrifugation. The cell pellet (paste) was stored at -70°C and refrigerated until use.
Embodiment 3
[0766] Purification of human Akt isoforms and ΔPH-Akt1
[0767] The cell pellet obtained from 1 L of S2 cells described in Example 2 was dissolved in 50 ml of buffer A (50 mM Tris pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.2 mM AEBSF, 10 μg / ml benzamidine) containing 1% CHAPS , 5 μg / ml each of leupeptin, aprotinin and pepstatin, 10% glycerol and 1 mM DTT), lysis was performed by sonication. The soluble fraction was purified with a protein G Sepharose (Sepharose) fast-flow (Pharmacia) column equipped with 9 mg / ml anti-intermediate T monoclonal antibody, and was purified with 75 μM EYMPME (SEQ.ID.NO.: 14) peptide in 25% Glycerol in Buffer A solution for elution. Fractions containing Akt / PKB were pooled and protein purity was assessed by SDS-PAGE. Purified proteins were quantified using standard Bradford protocols. Purified proteins were snap frozen in liquid nitrogen and stored at -70°C.
[0768] Requirement for activation of Akt and Akt platelet leukocyte C kinase substrate homology...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com