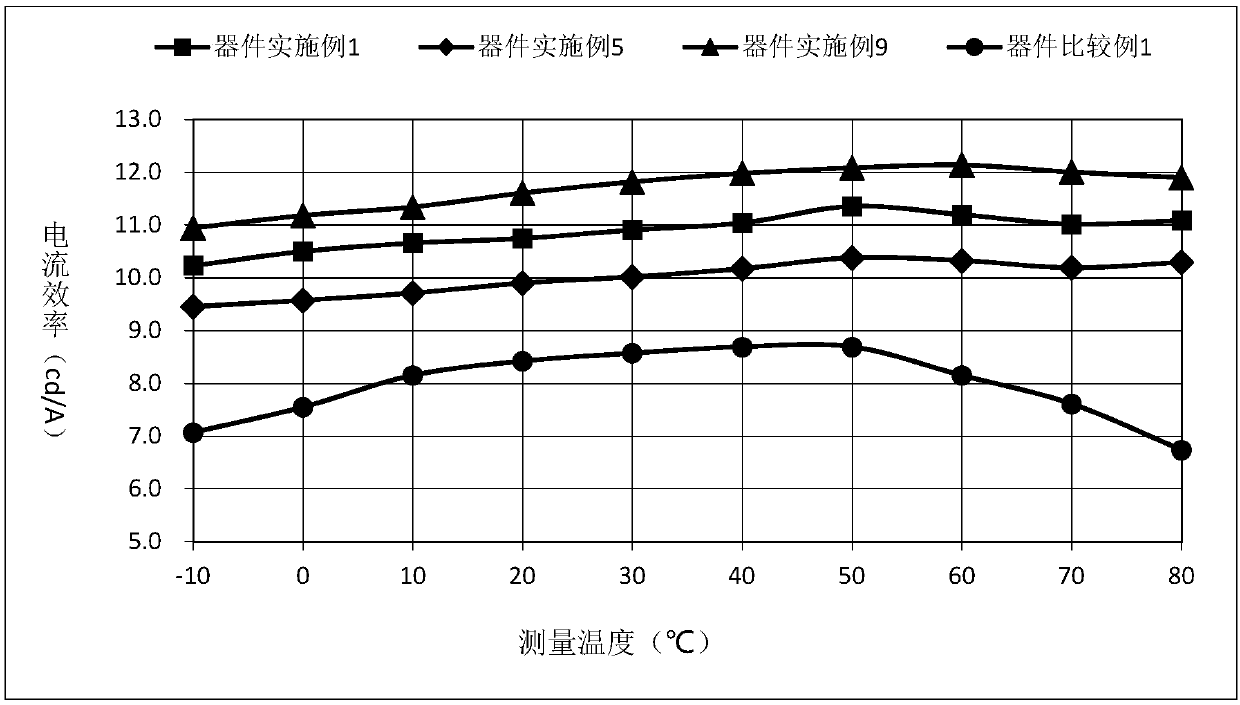
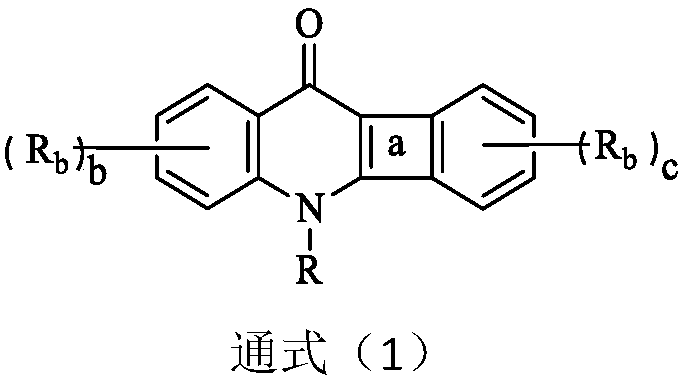
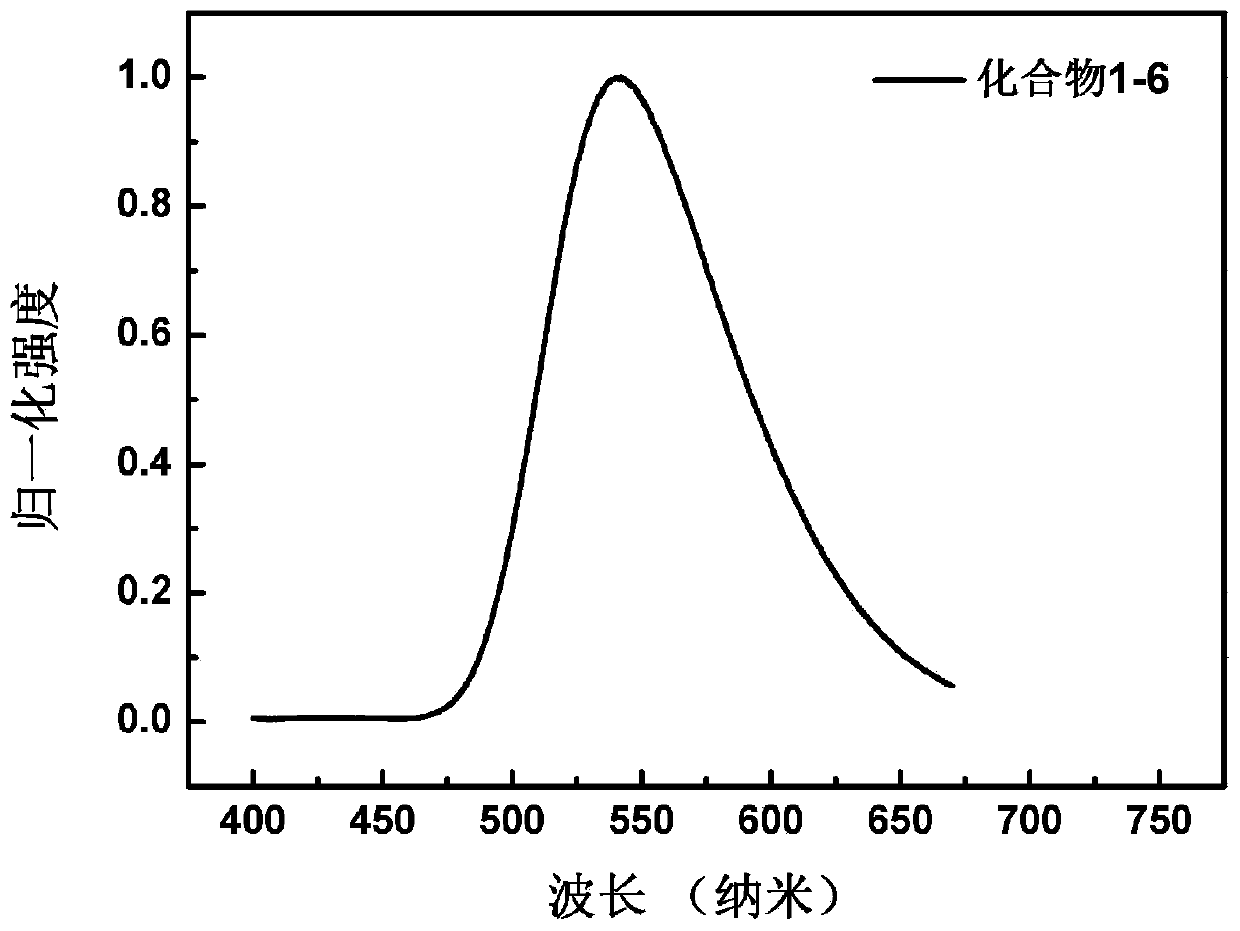
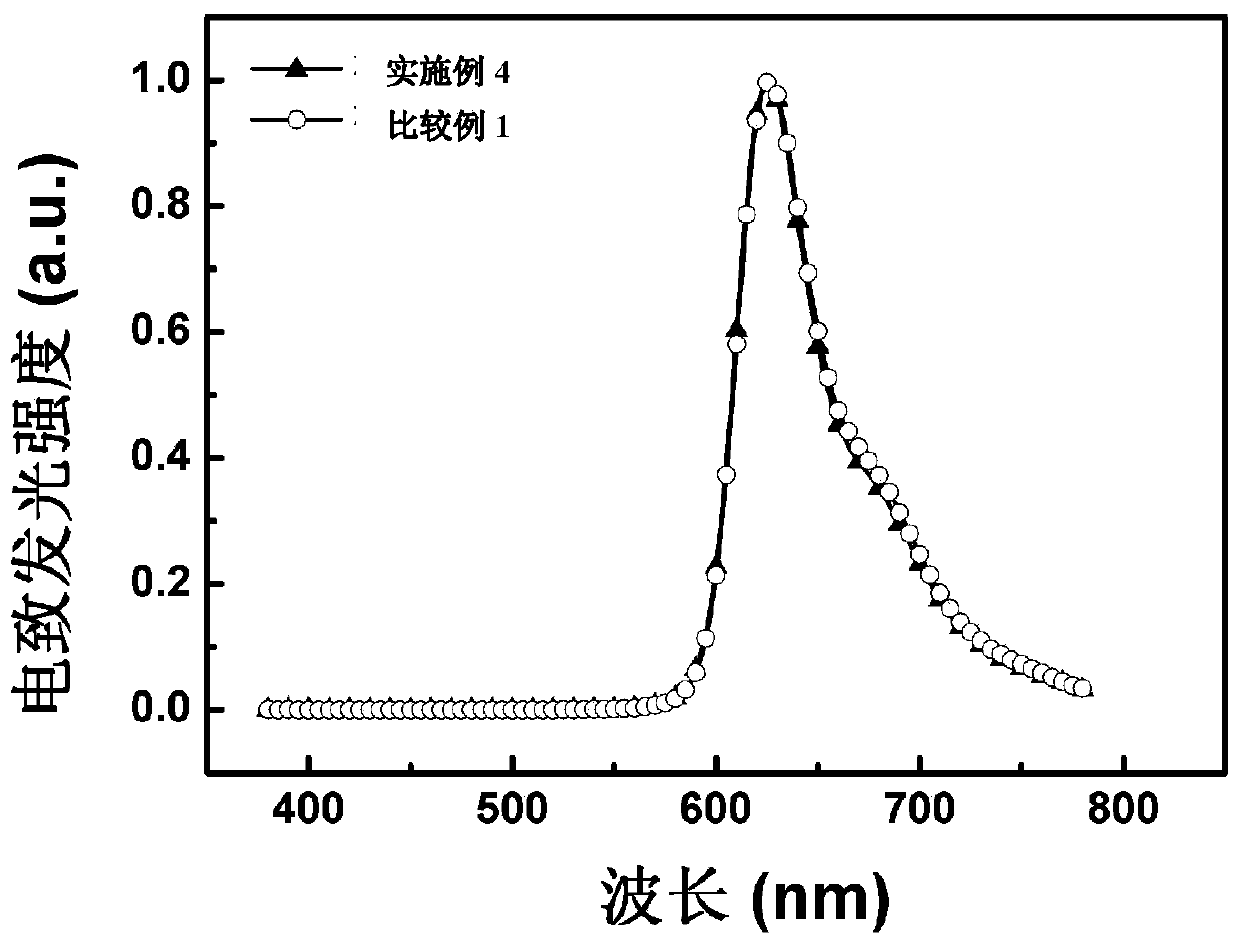
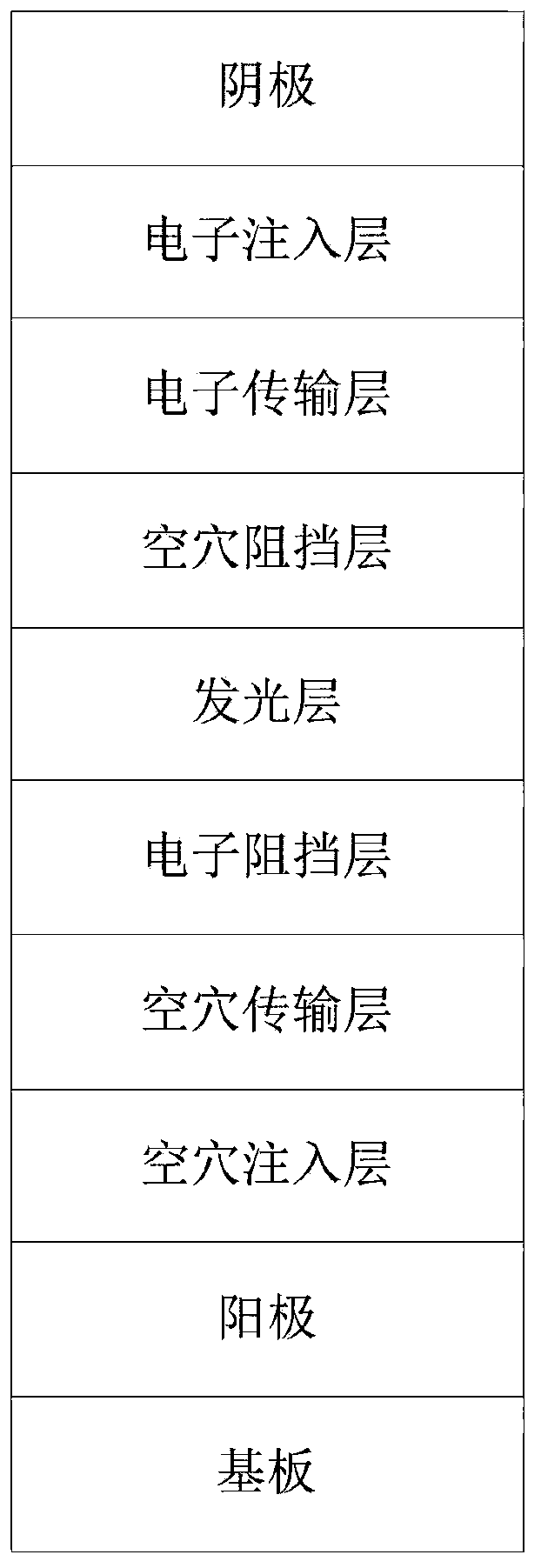
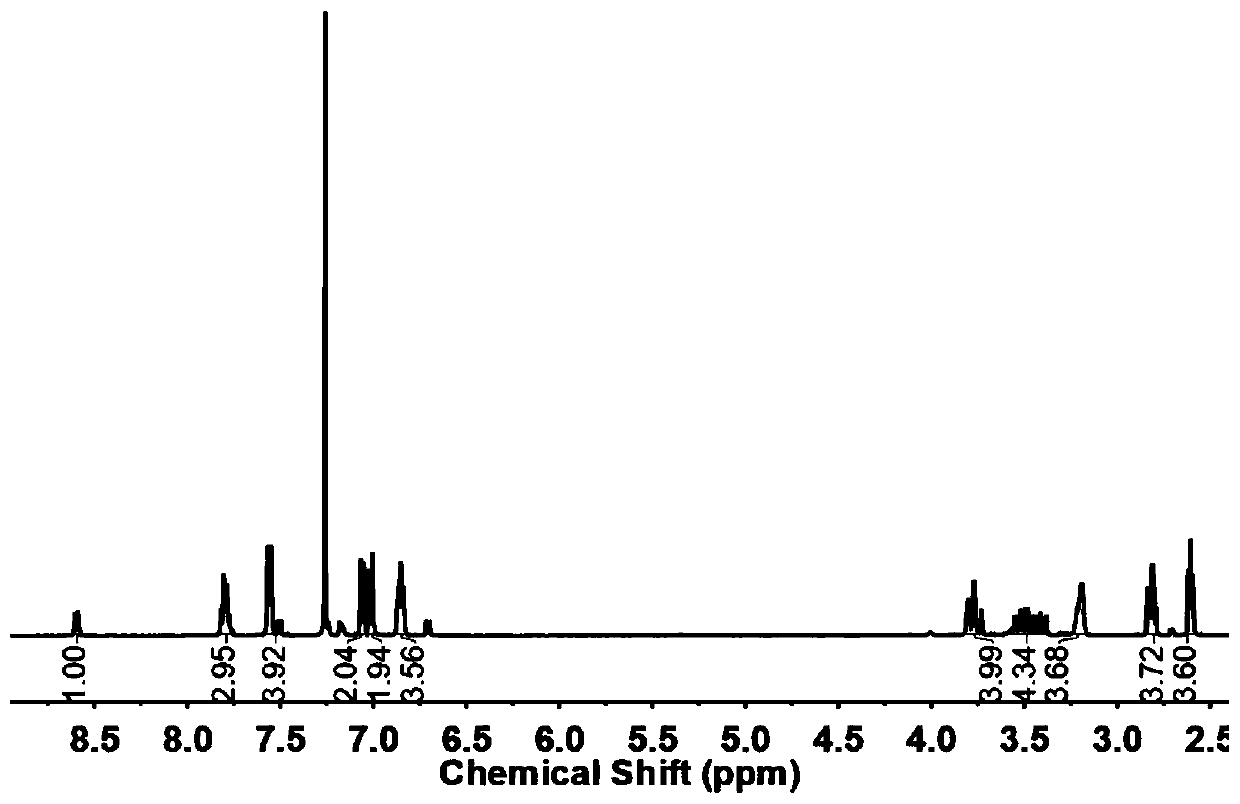
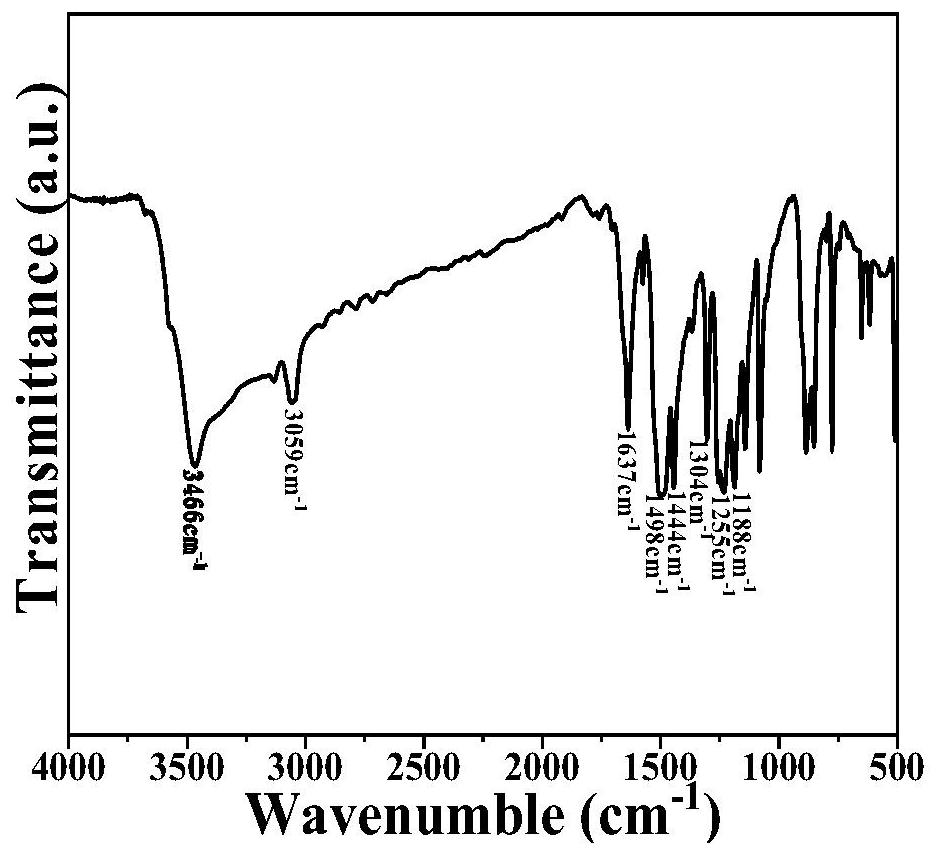
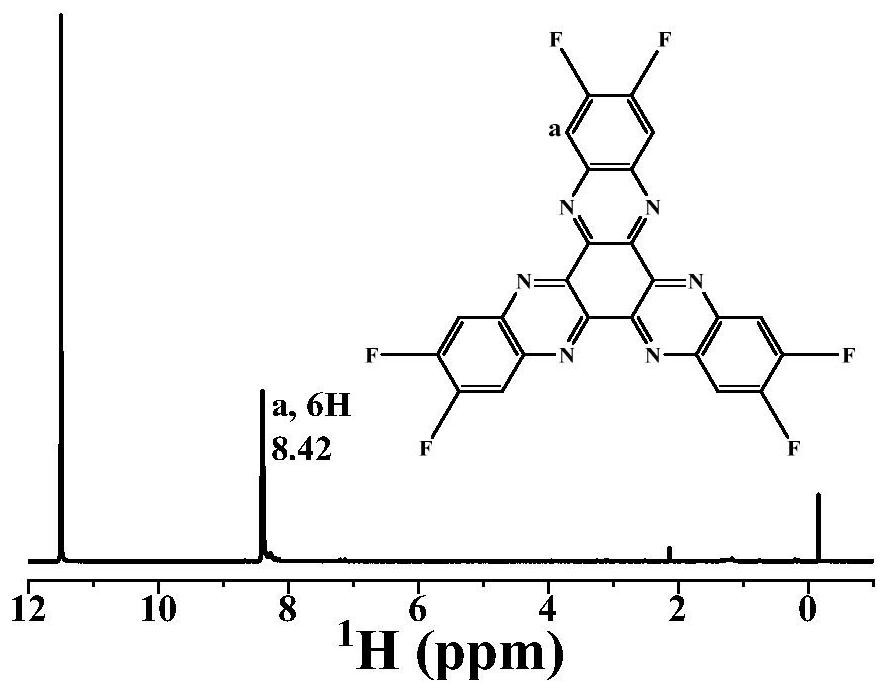
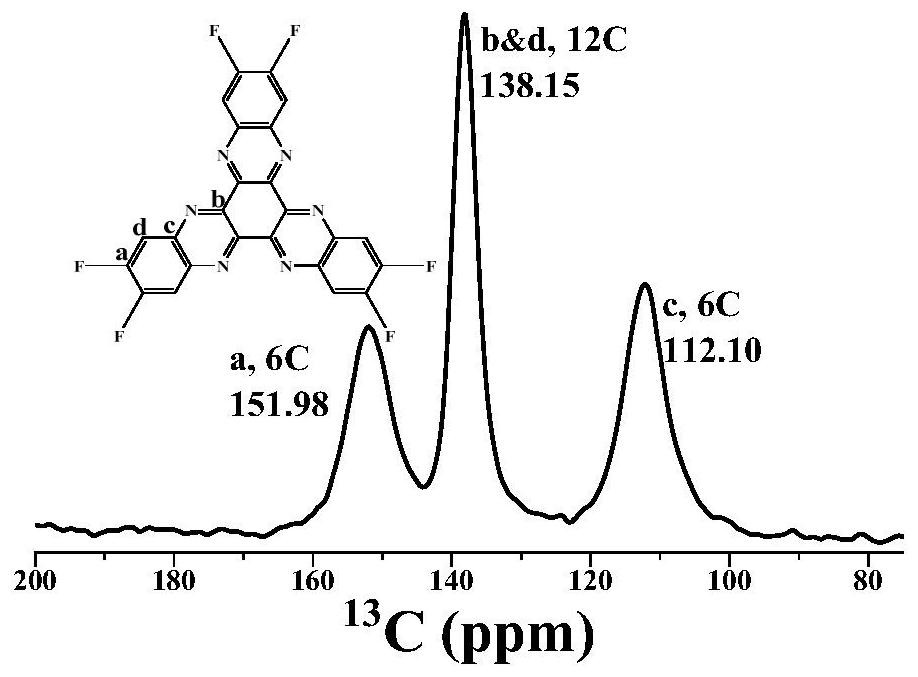
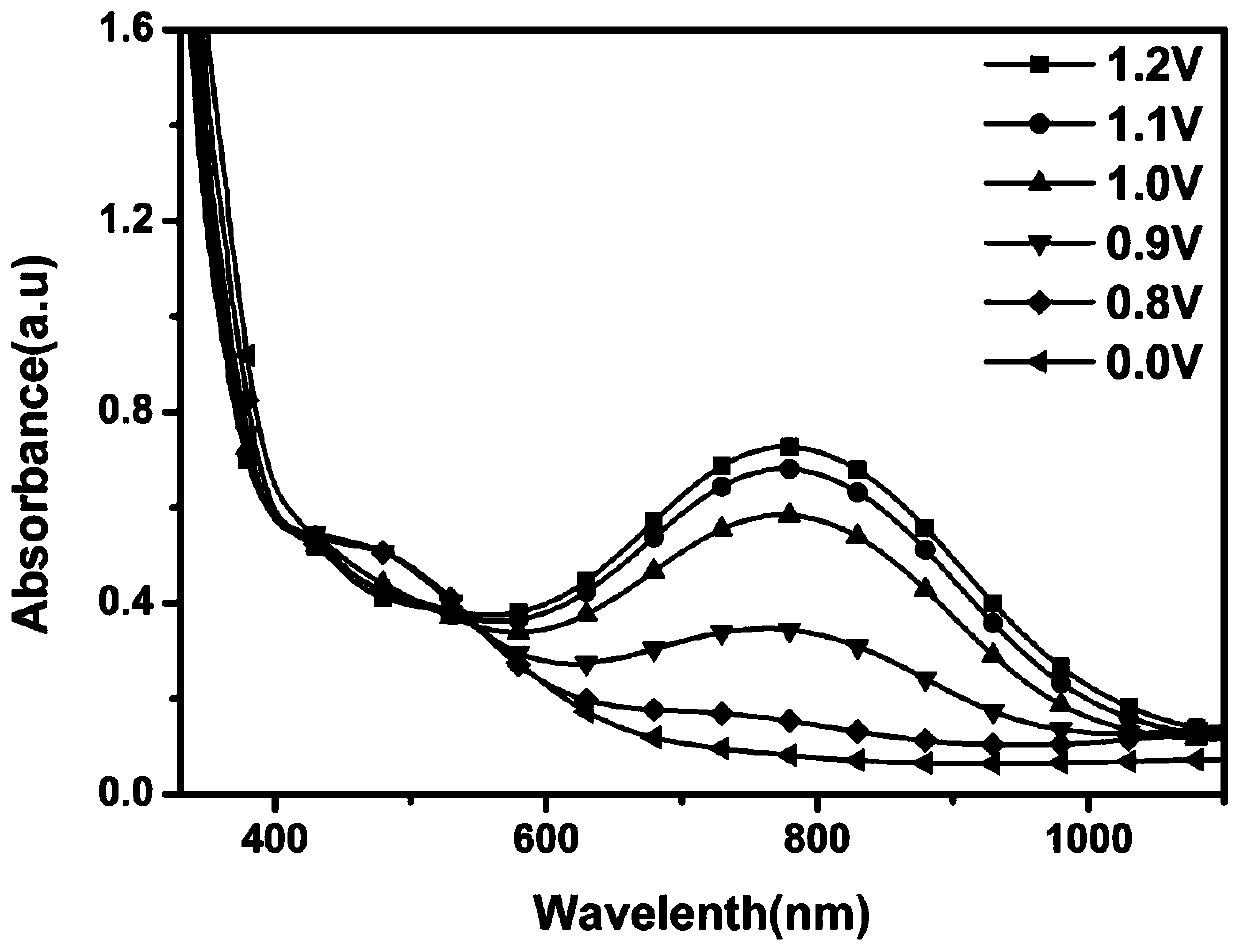
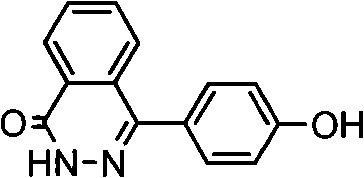
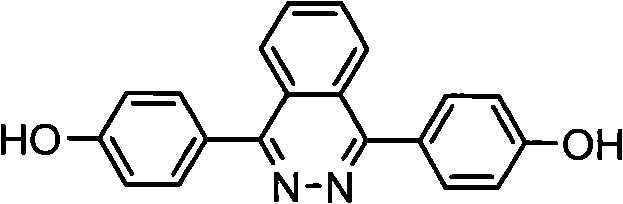
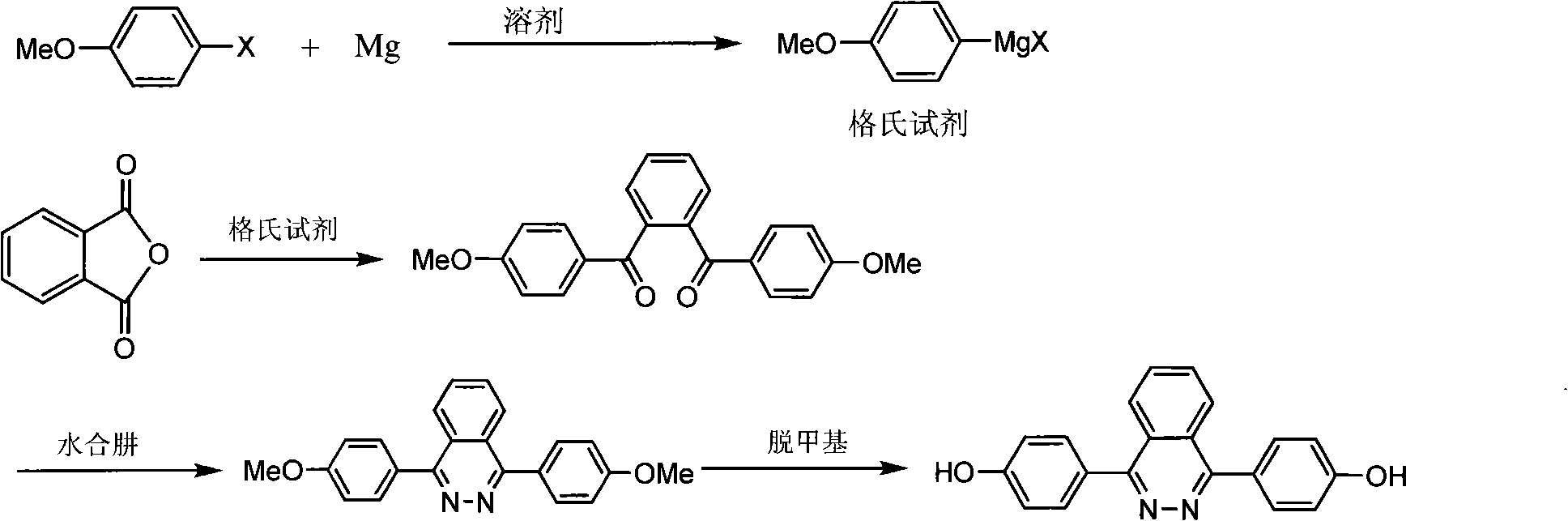
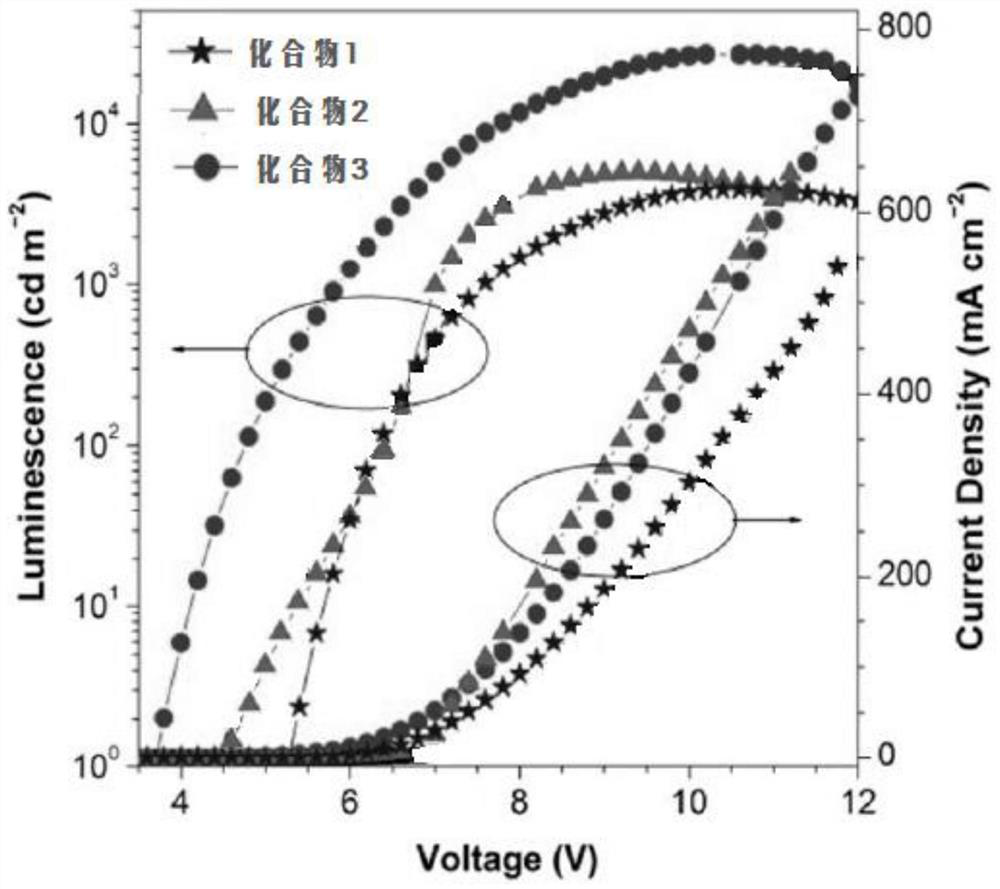
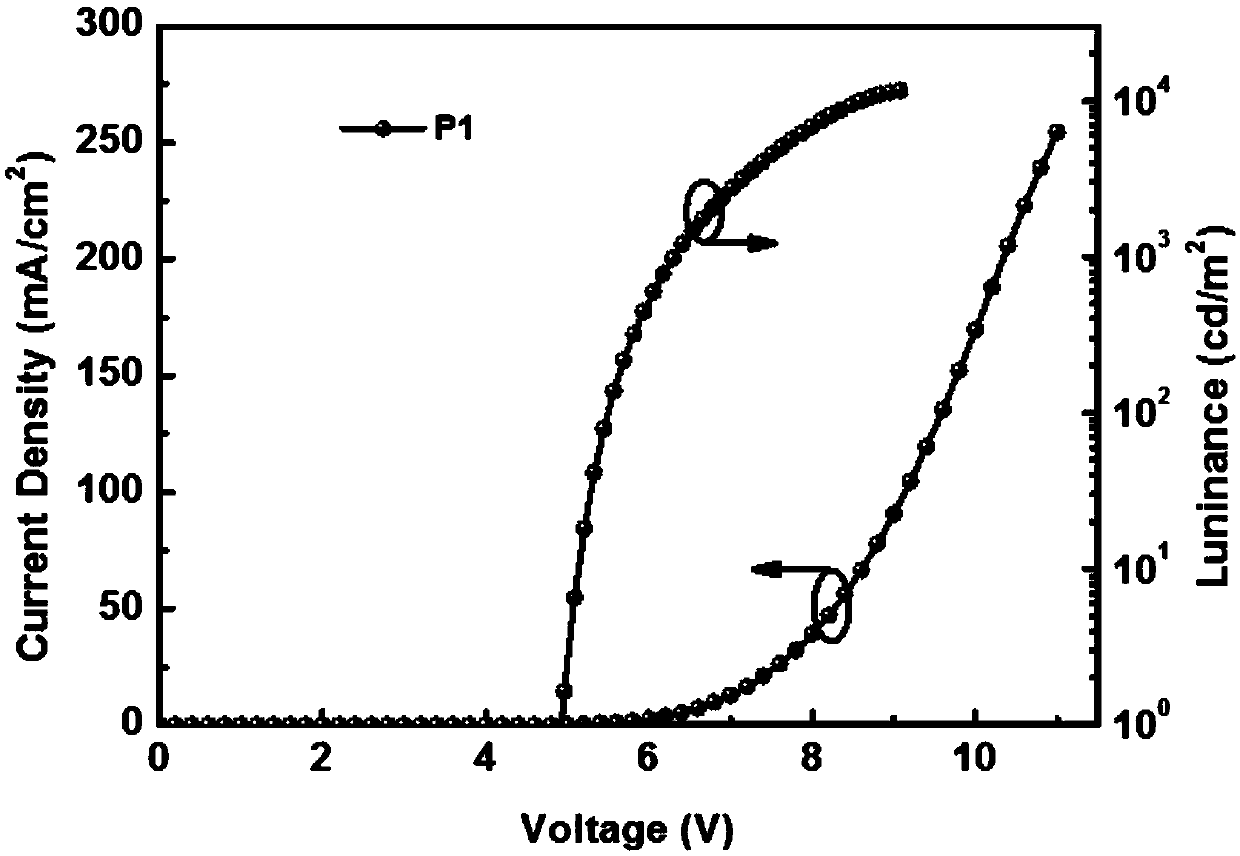
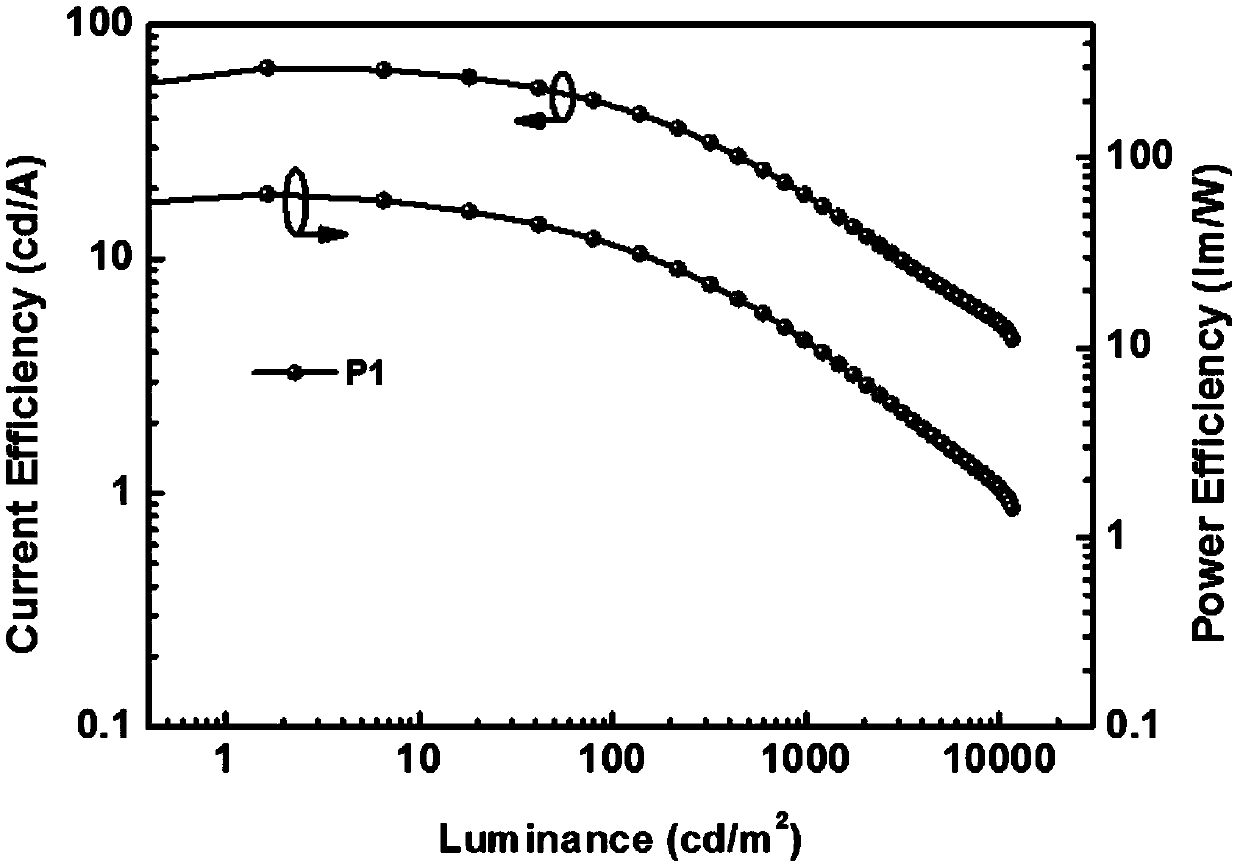
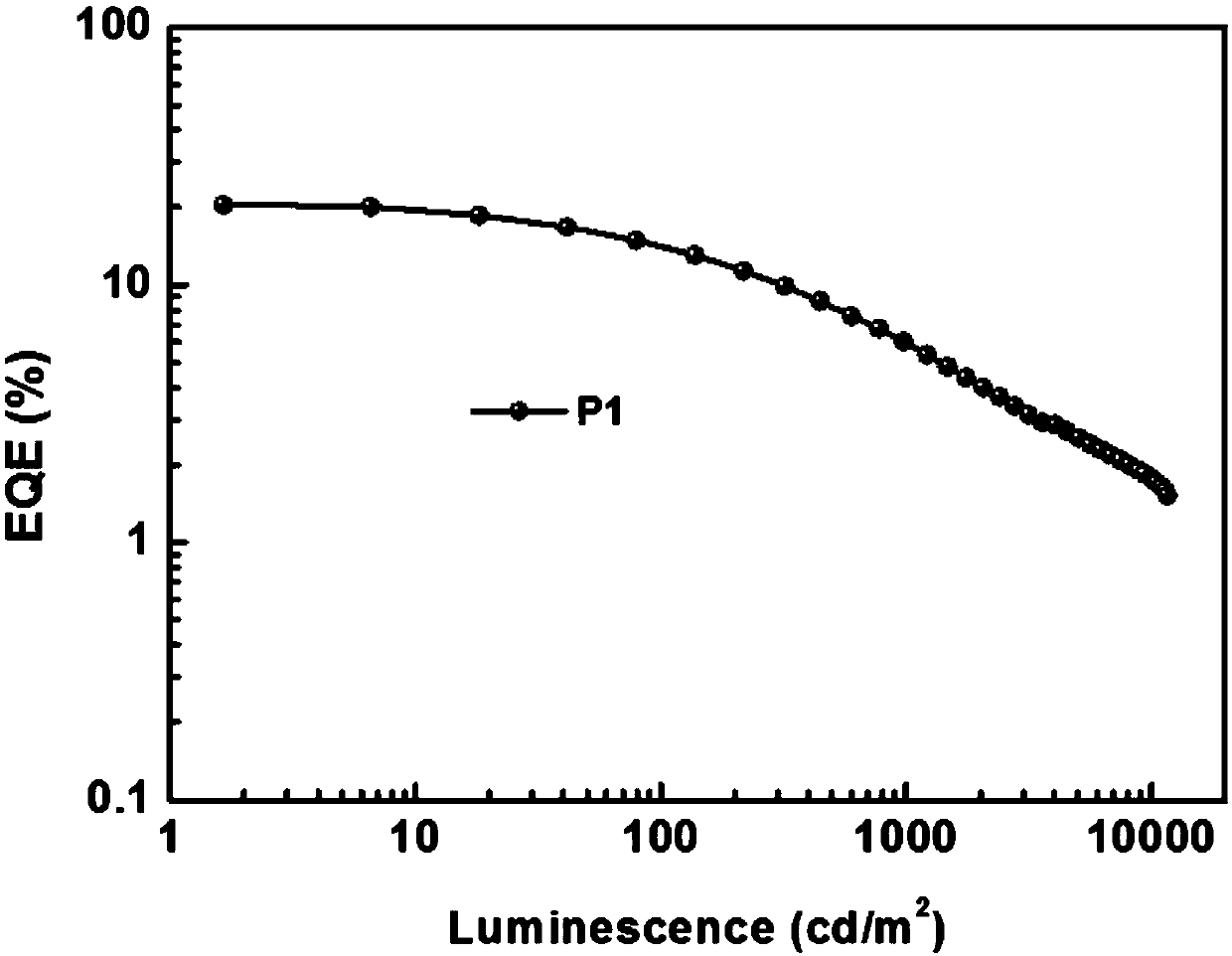
Patents
Literature
74 results about "Diazanaphthalene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diazanaphthalenes are a class of aromatic heterocyclic chemical compounds that have the formula C₈H₆N₂. They consist of a naphthalene double ring in which two of the carbon atoms have been replaced with nitrogen atoms. There are ten positional isomers, which differ by the locations of the nitrogen atoms.
1,8-Naphthyridine compound and organic light-emitting device using the same
InactiveUS20060286408A1Increase brightnessIncreased durabilityOrganic chemistryDischarge tube luminescnet screensDiazanaphthaleneTrifluoromethyl
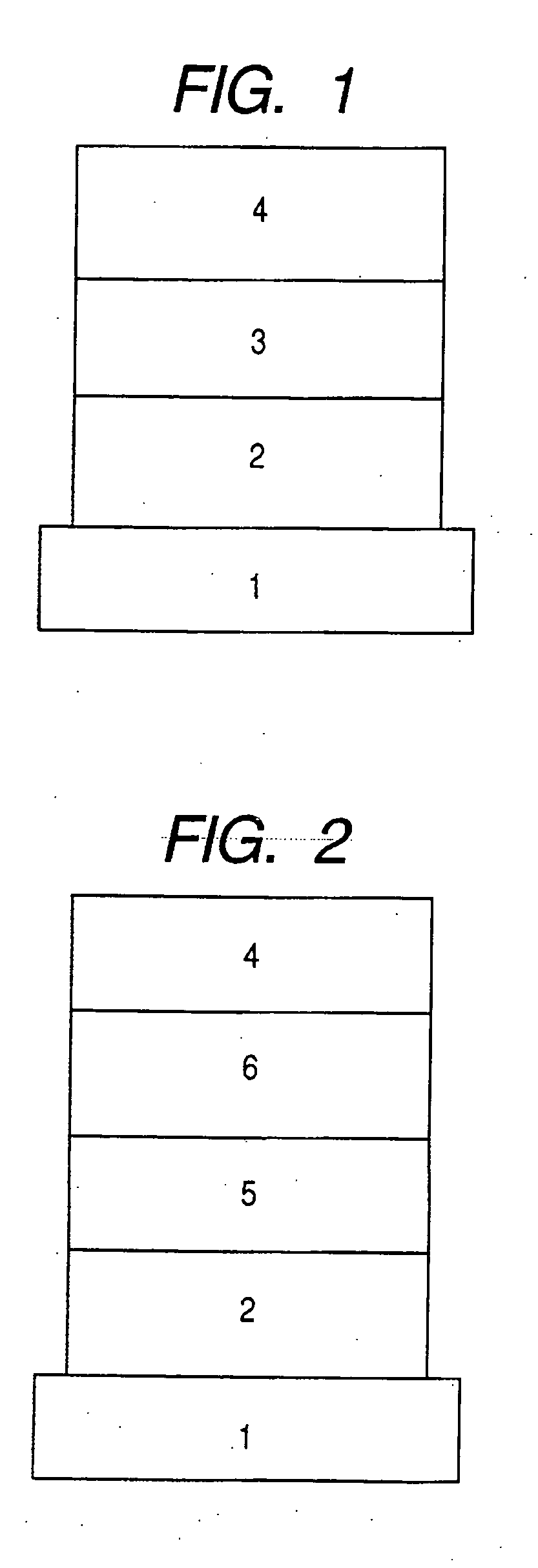
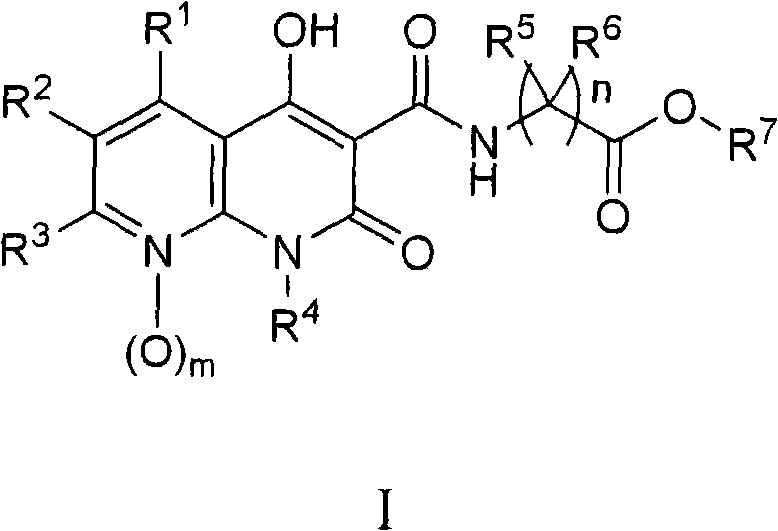
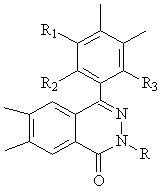
Provided a novel 1,8-naphthyridine compound represented by the following general formula [I]: wherein R1 to R6 each represent one selected from the group consisting of a hydrogen atom; an alkyl group, an aralkyl group, an aryl group, a heterocyclic group, a condensed polycyclic aromatic group, a condensed polycyclic heterocyclic group and an aryloxy group which may be substituted; a substituted amino group; a halogen atom; a trifluromethyl group; and a cyano group, and may be the same as or different from one another, provided that at least two of R1 to R6 each represent one selected from the group consisting of an aralkyl group, an aryl group, a heterocyclic group, a condensed polycyclic aromatic group, a condensed polycyclic heterocyclic group and an aryloxy group which may be substituted; and a substituted amino group.
Owner:CANON KK
Naphthyridine deratives or salts thereof
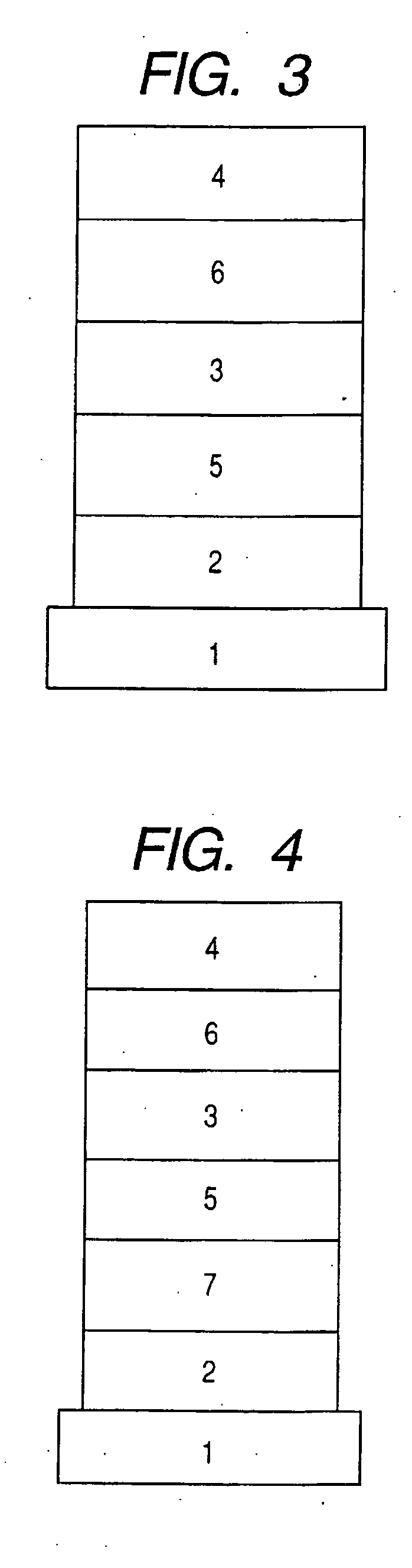
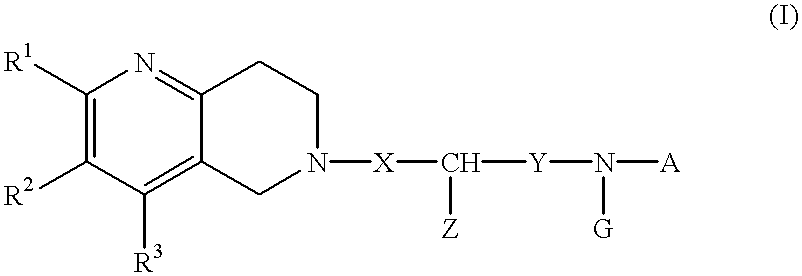
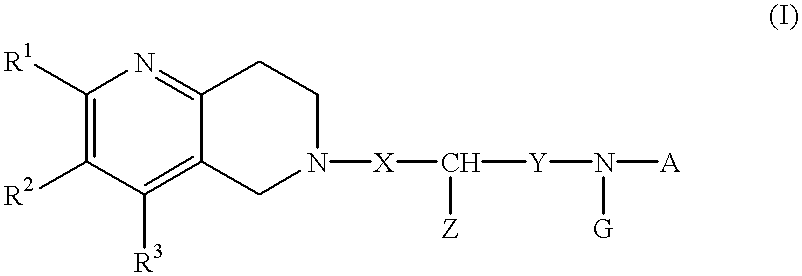
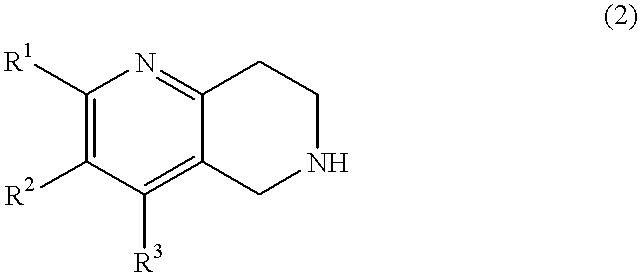
A novel naphthyridine compound represented by the general formula (1):wherein R1, R2 and R3 are independently a hydrogen atom, a lower alkyl group or the like, or R1 and R2, or R2 and R3, when taken together, form a cyclic group; each of X and Y is a methylene or ethylene group; Z is a phenyl group, a substituted phenyl group or the like; A is a hydrogen atom, a lower alkyl group or the like; and G is an acyl group, shows antagonism for tachykinin receptors and is useful as a prophylactic or therapeutic agent for diseases for which the tachykinin receptors are considered to be responsible. A specific example of such a compound is 2-[(-)-4-(N-benzoyl-N-methyl)amino-3-(3,4-dichlorophenyl)butyl]-10-acetylamino-1,2,3,4-tetrahydro-benzo[b][1,6]naphthyridine.
Owner:NIPPON KAYAKU CO LTD
Method of preparing diazanaphthalene biphenyl type sulphonation polyarylethernitrile electrolyte film material
InactiveCN101016375AHigh strengthImprove toughnessSolid electrolyte fuel cellsCollectors/separatorsAlkaline earth metalSwelling ratio
The invention discloses a preparing method of two aza naphthalene biphenyl type sulfonated polyarylether nitrile ion conductive film material, which comprises the following steps: incorporating structural element of formula 1; passing two aza naphthalene ketone biphenyl bis-phenol, dihalogen (or two nitro group) cyanobenzene, 3, 3'-two petroleum sulfonate-4, 4'- aromatic dihalogen (or two nitro group) and aromatic dihalogen (or two nitro group) as monomer; choosing alkali or salt of alkali metal or alkali earth metal; getting the product in non-proton polar solvent through high-heat copolymerization reaction; wherein R1, R2, R3, R4 maybe alkyl group, alkoxy, phenyl group, halogen atom or hydrogen atom. This invention possesses good tenacity, dig intensity, low swelling ratio, which can be used to fuel battery or energy-storage battery or film separating technology domain.
Owner:DALIAN UNIV OF TECH
Novel block anion exchange membrane and preparation method thereof
InactiveCN105906812AHigh conductivity free volume increaseIncreased free volumeFuel cellsPolymer scienceFuel cells
The invention discloses a novel block anion exchange membrane. A block main chain of the anion exchange membrane contains a bending unit (biphenyl fluorene) and a twisting unit (phthalazinone); according to the structure, a non-coplanar effect can be enhanced. A preparation method of the anion exchange membrane comprises the following steps of performing polycondensation on decafluorobiphenyl (slightly excessive) and bisphenol fluorine to prepare an oligomer 1; using the decafluorobiphenyl and 4-(4-hydroxyphenyl)-2,3-phthalazine-1-one to prepare an oligomer 2; polymerizing the oligomer 1 with the oligomer 2 to obtain a polymer main chain, and performing ionization treatment, membrane casting and alkali treatment on a chloromethylation main chain to obtain the anion exchange membrane. The anion exchange membrane has better chemical stability, and higher electric conductivity and resistance to swelling, and is suitable for being applied in the aspect of alkaline fuel cells.
Owner:DALIAN UNIV OF TECH
Novel 1,8-naphthyridine compounds
The present invention relates to naphthyridine compounds useful as HIF prolyl hydroxylase inhibitors to treat anemia and like conditions.
Owner:MERCK & CO INC
Compound taking quinolinone derivative as core and application of compound in organic electroluminescent device
ActiveCN110964007AIncrease overlapReduce energy level differenceOrganic chemistrySolid-state devicesElectron holeElectron donor
The invention discloses a compound taking a quinolinone derivative as a core and an application of the compound in an organic electroluminescent device. The compound structurally contains the quinolinone derivative as an electron acceptor, so that transmission of electrons in a light-emitting layer is facilitated. A connected heterocyclic group is an electron donor, so as to facilitate the transmission of holes in the light-emitting layer. Nitrogen atoms in the heteroatom-containing benzoazadione are saturated atoms, have quite strong rigidity and are beneficial to improving the triplet stateenergy level of the parent nucleus compound, and the combination of the electron donor and the electron acceptor can improve the recombination efficiency of excitons, the starting voltage is reduced and the performance of the device is improved. When the compound is used as a light-emitting layer material of the organic electroluminescent device, the current efficiency of the device is greatly improved, and the service life of the device is obviously prolonged.
Owner:JIANGSU SUNERA TECH CO LTD
Network polymer material applied to super capacitor electrode and provided with nitride and oxygen atoms and preparation method thereof
ActiveCN106188539AGood electrochemical propertiesHigh specific capacitanceHybrid capacitor electrodesCapacitanceOxygen
The invention discloses a network polymer material applied to a super capacitor electrode and provided with nitride and oxygen atoms and a preparation method thereof and belongs to the field of material science. The network polymer material provided with the nitride and oxygen atoms is a network polymer with phthalazone and s-triazine structures and has a repetitive unit structure expressed as formula I. The preparation method of the network polymer material comprises the following steps: step one, taking a halogen compound with a phthalazone structure as the raw materials and carrying out coupling reaction to generate a dinitrile monomer; and step two, taking the dinitrile monomer as the raw material and carrying out polymerization reaction to generate the network polymer with phthalazone and s-triazine structures. A super capacitor taking the material as the electrode has the characteristics of high specific capacitance and excellent cycling stability; when the current density is 0.1A / g, the specific capacitance is 302F / g; the specific capacitance of the super capacitor is free of attenuation after 30000 charging and discharging cycles.
Owner:DALIAN UNIV OF TECH +1
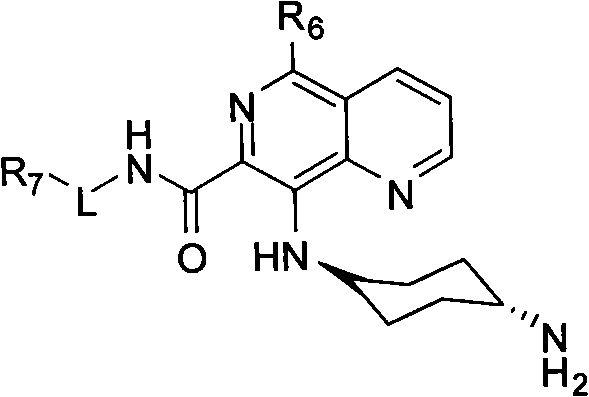
Maleate salts of B-RAF kinase inhibitor, crystalline forms, methods of preparation, and uses therefore
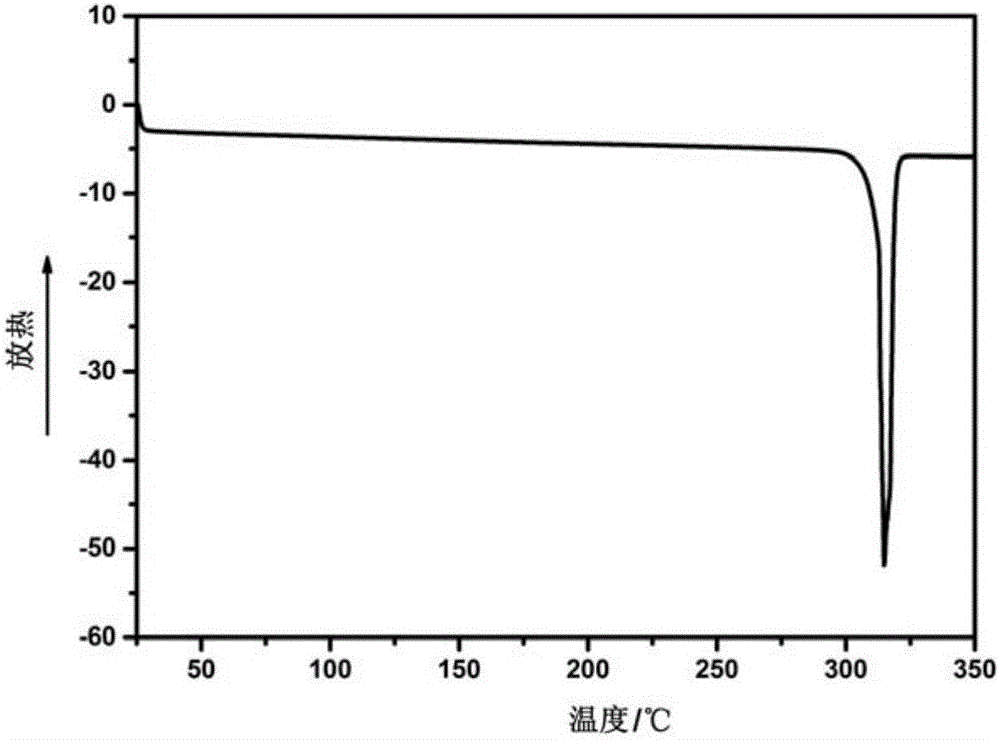
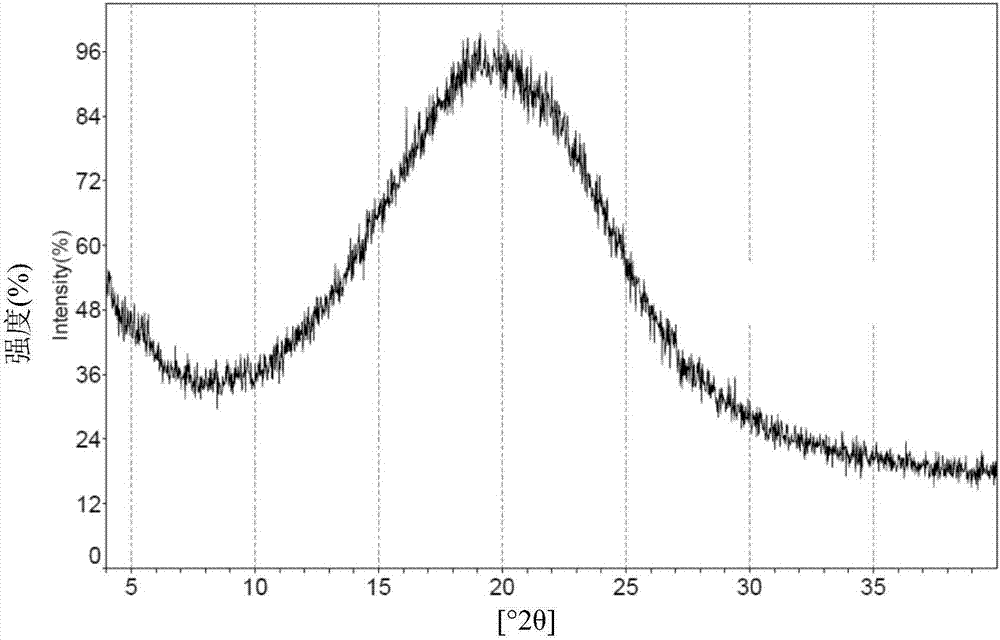
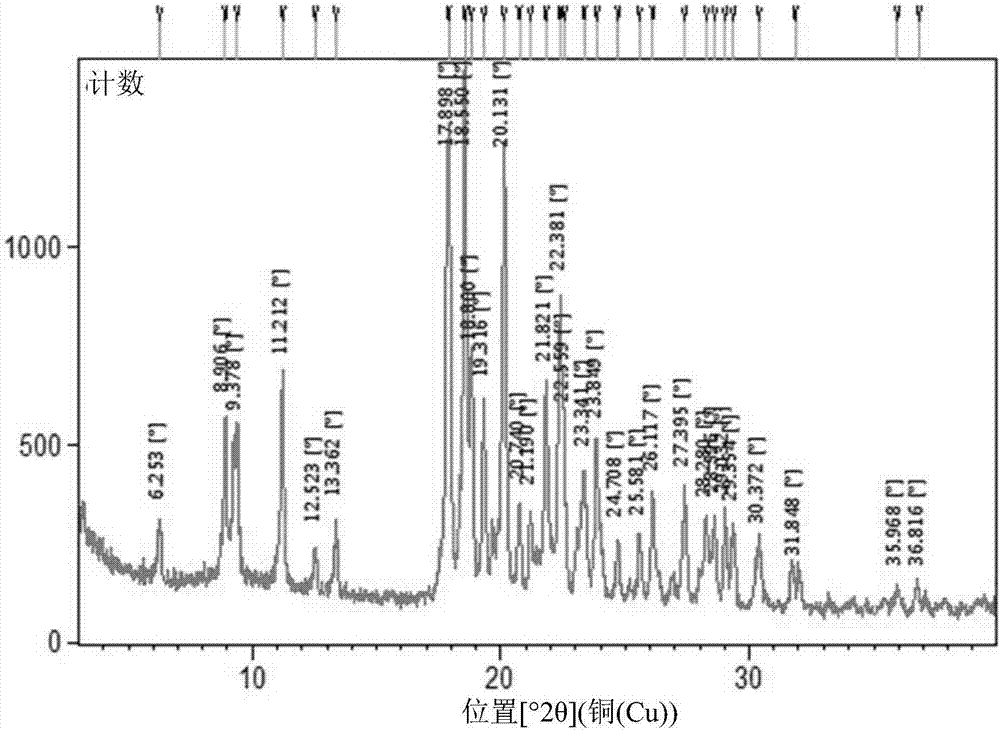
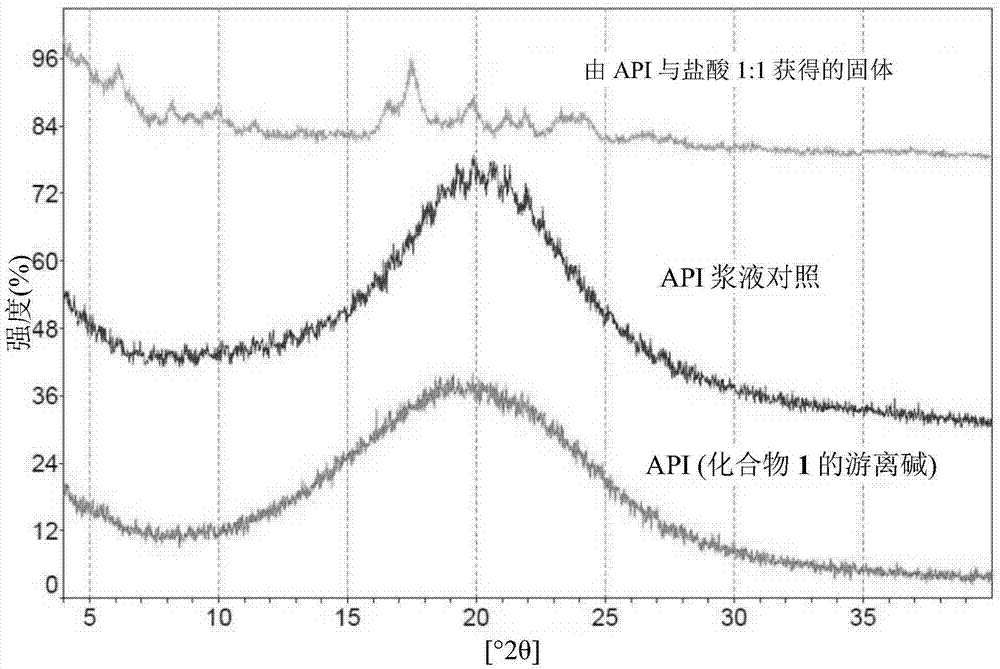
The invention relates to 5- ( ( (1R, 1aS, 6bR) -1- (6- (trifluoromethyl) -1H-benzo [d] imidazol-2-yl) -1a,6b-dihydro-1H-cyclopropa [b] benzofuran-5-yl) oxy) -3, 4-dihydro-1, 8-naphthyridin-2 (1H) -one(Compound 1) maleate salts, in particular the sesqui-maleate salt and its crystalline forms, methods of preparation, pharmaceutical compositions, and therapeutic uses for treatment of diseases or disorders mediated by BRAF or other kinases.
Owner:BEIGENE LTD
Novel compounds
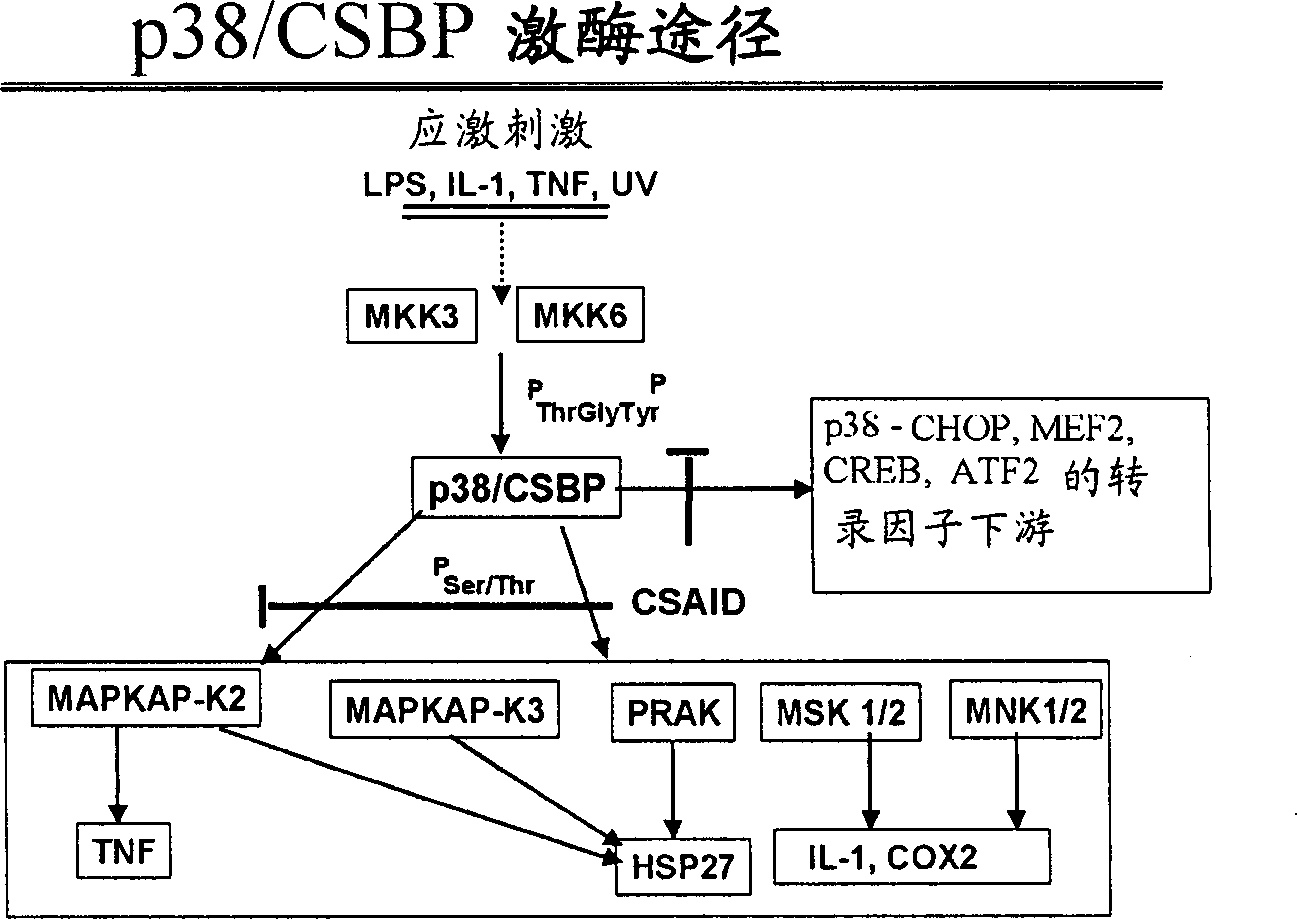
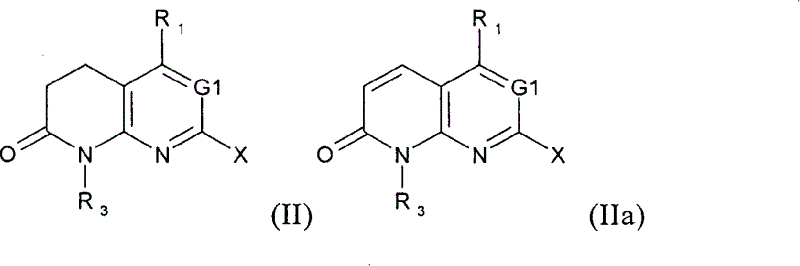
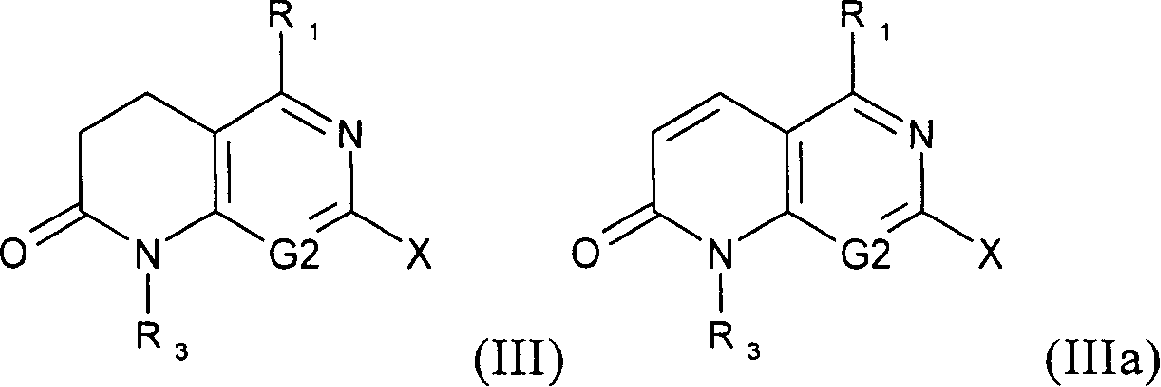
New substituted 1,5,7-trisubstituted-1,8-naphthyridine-2(1H)-one compounds; 1,5,7-trisubstituted-1,6-naphthyridine-2 -(1H)-one compounds and 1,5,7-trisubstituted quinolin-2(1H)-one compounds, their preparation methods, their use in the treatment of CSBP / p38 kinase-mediated diseases, and for A pharmaceutical composition for the treatment.
Owner:SMITHKLINE BECKMAN CORP
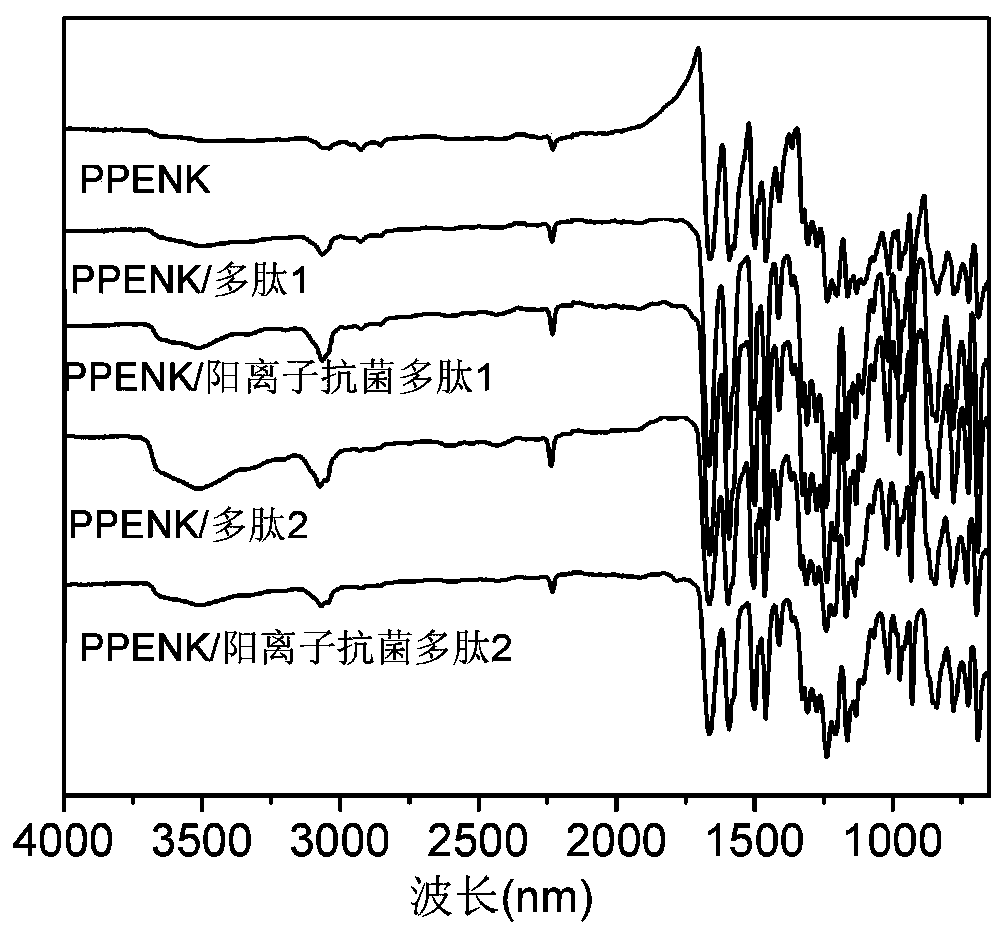
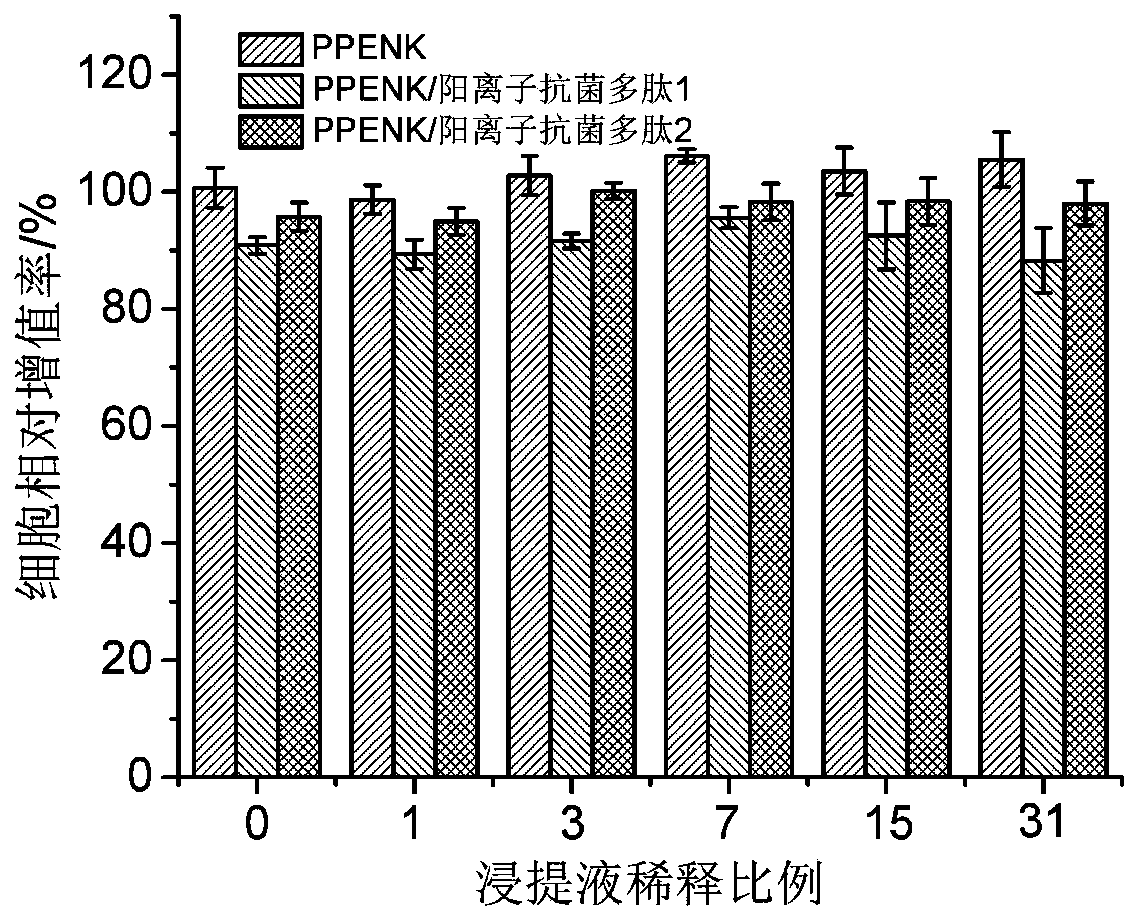
Poly(phthalazinone ether nitrile) having antibacterial properties, and surface modification method of poly(phthalazinone ether nitrile) having antibacterial properties
ActiveCN110585482AGood biocompatibilityHigh antibacterial activityPharmaceutical delivery mechanismCoatingsIonDiazanaphthalene
The invention belongs to the field of medical high molecular biomaterials, and particularly relates to a surface antibacterial and modified polyarylene ether nitrile antibacterial material containinga diazanaphthalene terphenyl structure, and a preparation method of the surface antibacterial and modified polyarylene ether nitrile antibacterial material containing a diazanaphthalene terphenyl structure. The material is prepared through performing chemical bonding of an antibacterial activity coating on the plane surface or a three-dimensional surface of poly(phthalazinone ether nitrile), wherein the antibacterial activity coating comprises a cation antibacterial polypeptide layer having antibacterial activity, and cation antibacterial polypeptide is fixed to the surface of the poly(phthalazinone ether nitrile) by a chemical bonding method. Under the premise that the mechanical properties of the polyarylene ether nitrile are not influenced, the biocompatibility of the polyarylene ethernitrile material can be improved, the polyarylene ether nitrile material has the antibacterial properties, the preparation method is simple, and the polyarylene ether nitrile material is suitable forbeing applied to the field of biologic medical materials.
Owner:DALIAN UNIV OF TECH
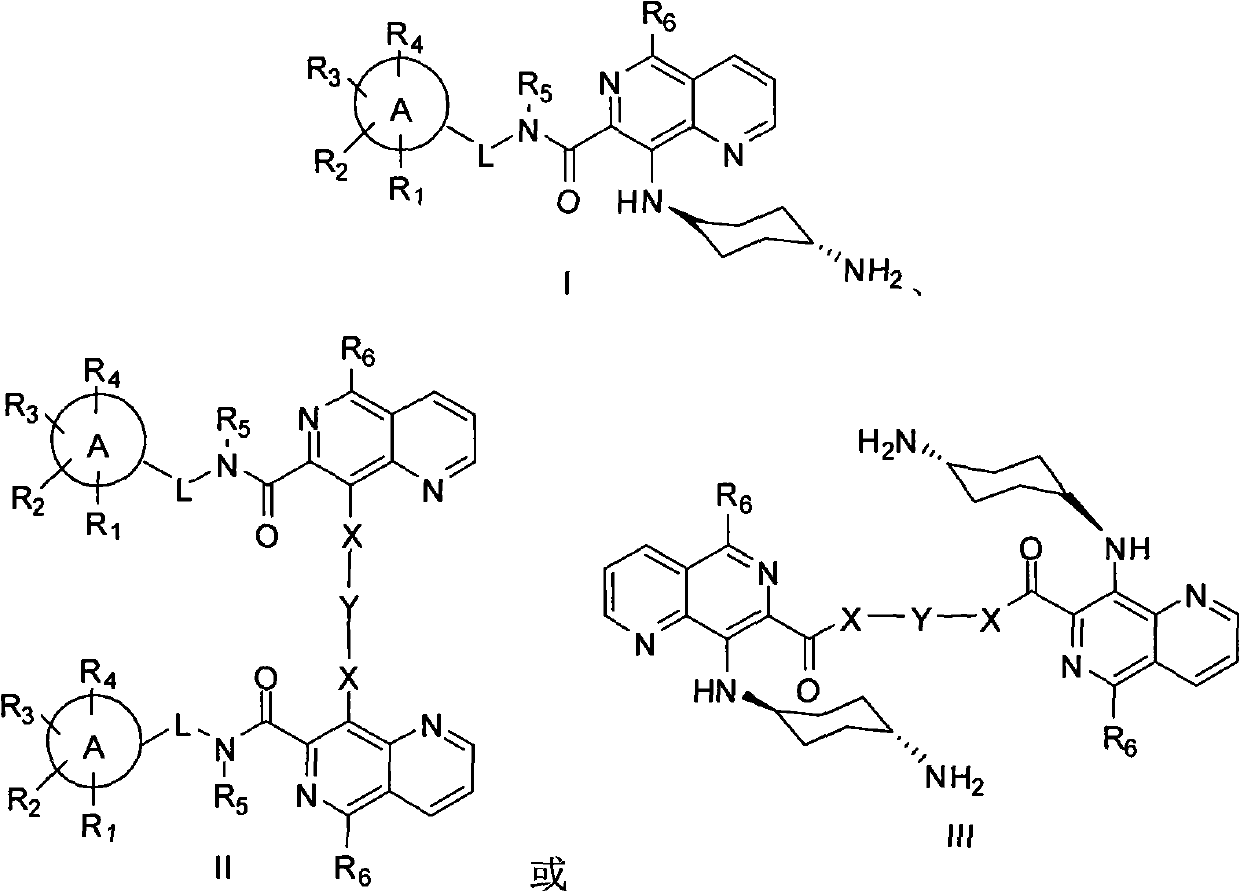
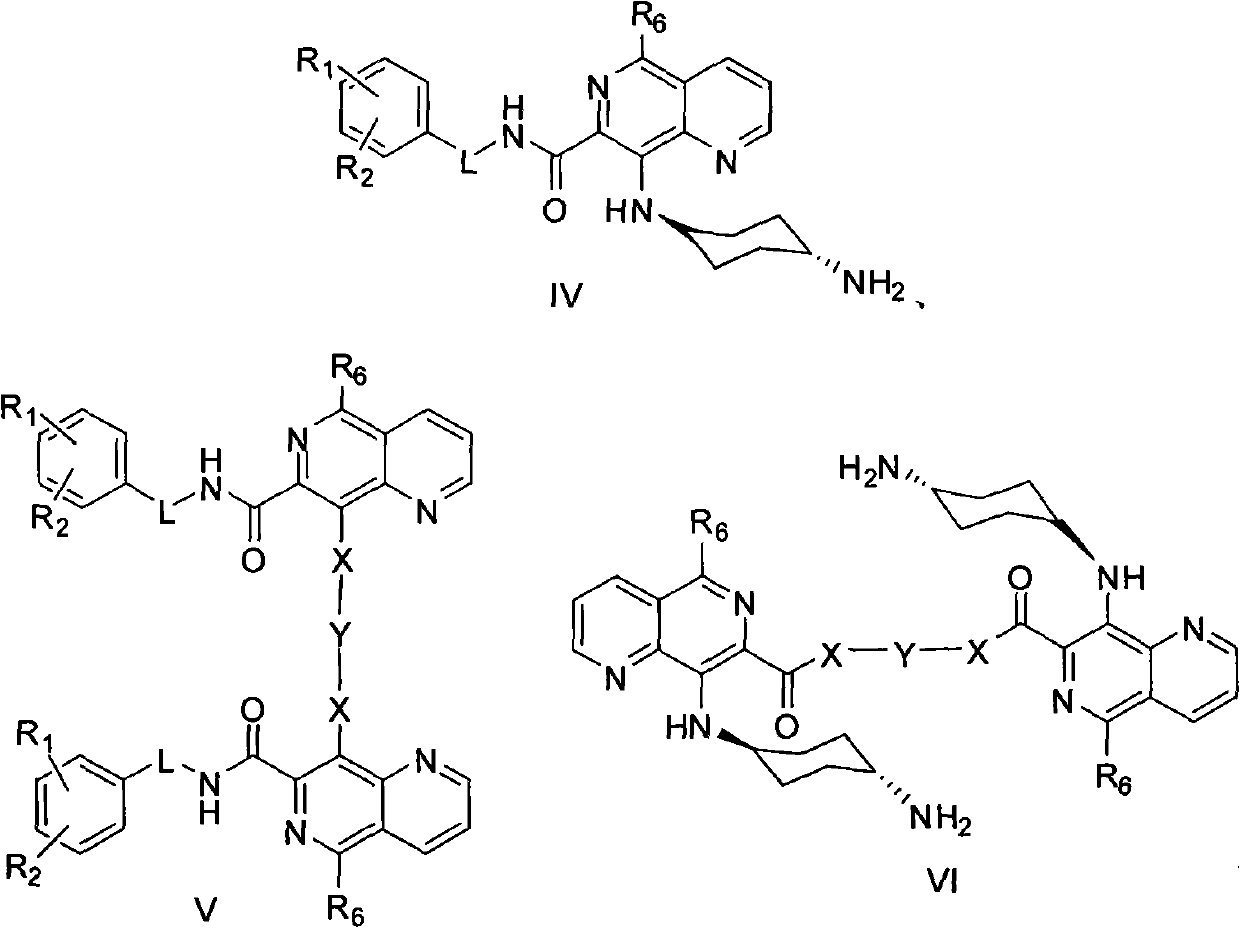
5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, dimer compounds of 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, and preparation method and use of 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds and dimer compounds of 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds
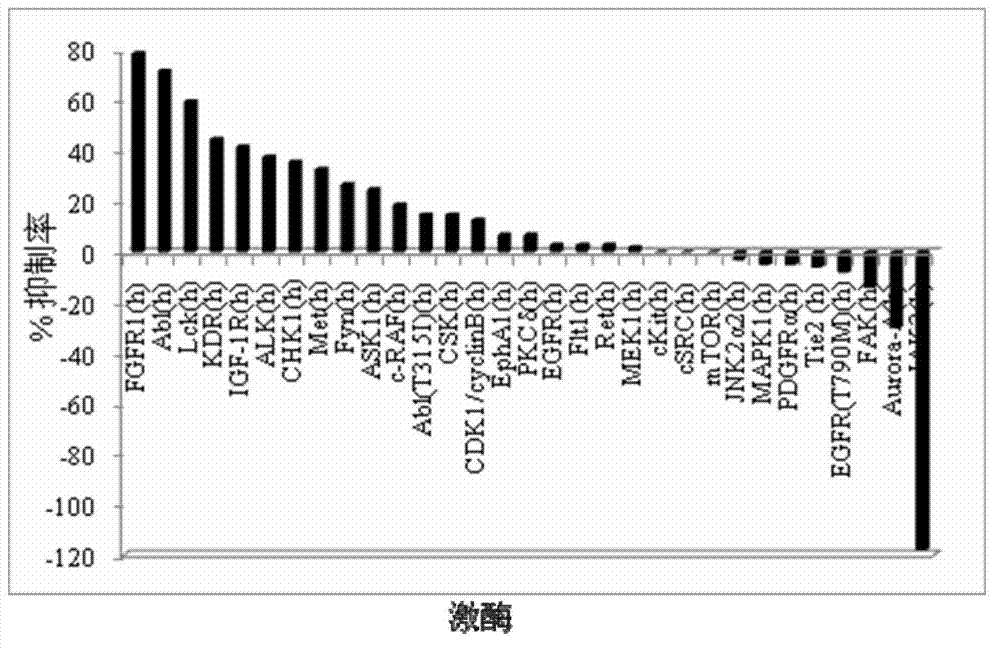
InactiveCN102558172ABroaden the structure typeStrong inhibitory activityOrganic active ingredientsOrganic chemistryTyrosine Protein KinasesProtein-Tyrosine Kinases
The invention discloses 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, dimer compounds of the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, a preparation method and use of the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds and the dimer compounds of the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, and pharmaceutical compositions containing the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds. More specifically, the invention discloses the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds expressed by structural formulae I and II or III, the dimer compounds of the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds and the preparation method of the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds and the dimer compounds of the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, and provides the use of the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds and the pharmaceutical compositions containing the 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds in the treatment of diseases related to protein tyrosine kinase, in particular to c-Src, such as tumor diseases, by serving as a multi-target spot protein tyrosine kinase inhibitor.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Preparation method of diaza-naphthalenone-biphenyl-polybenzoxazole, monomer and polymer
InactiveCN102585225AImprove heat resistanceImprove mechanical propertiesOrganic chemistryFiberPolymer science
The invention belongs to the field of high performance macromolecule materials. Diaza-naphthalenone-biphenyl-polybenzoxazole utilizes a monomer with a diaza-naphthalenone-biphenyl structure. The diaza-naphthalenone-biphenyl-polybenzoxazole or a polymer of the diaza-naphthalenone-biphenyl-polybenzoxazole is obtained by adding the monomer and various diacid or acid halide of the diacid into polyphosphoric acid, adding or not adding other salt of diamido-diphenol and conducting high temperature solution polycondensation reaction on the mixture. The monomer with the diaza-naphthalenone-biphenyl structure is stable in performance, does not need to be salified, can save a degassing step in polymerization, and greatly shortens polymerization time. The polymer has excellent heat resistance, good mechanical performance, abrasion resistance, radiation resistance, optical-electrical characteristics and improves dissolubility and can be widely used in fields such as high heat resistant fiber, resin, composite materials, films and coatings of the fiber, biological materials, adhesives and high performance optical-electrical materials.
Owner:DALIAN UNIV OF TECH
Polycyclic aromatic hydrocarbon aza-naphthalene derivative, synthesis method and electronic device thereof
PendingCN111548350AGood film formingImprove thermal stabilityOrganic chemistrySolid-state devicesOrganic solar cellOrganic field-effect transistor
The invention relates to the technical field of organic photoelectric materials, in particular to a polycyclic aromatic hydrocarbon aza-naphthalene derivative, a synthesis method thereof and an electronic device containing the polycyclic aromatic hydrocarbon aza-naphthalene derivative represented by a general formula (1), wherein Z represents CR1 or N. According to the polycyclic aromatic hydrocarbon aza-naphthalene derivative disclosed by the invention, a polycyclic aromatic hydrocarbon aza-naphthalene rigid structure is introduced, so that the obtained polycyclic aromatic hydrocarbon aza-naphthalene derivative is excellent in film-forming property and thermal stability, and can be used for preparing an organic light-emitting device, an organic field effect transistor and an organic solarcell. In addition, the polycyclic aromatic hydrocarbon aza-naphthalene derivative can be used as a constituent material of a hole injection layer, a hole transport layer, a light emitting layer, an electron blocking layer, a hole blocking layer or an electron transport layer, and can reduce the driving voltage, improve the efficiency, improve the brightness, prolong the service life and the like.
Owner:SUZHOU JOYSUN ADVANCED MATERIALS CO LTD
High-temperature-resistant and flame-retardant tetrafunctional epoxy resin with diazaphthone structure and preparation method of tetrafunctional epoxy resin
The invention belongs to the technical field of macromolecule science and provides high-temperature-resistant and flame-retardant tetrafunctional epoxy resin with a diazaphthone structure and a preparation method of the tetrafunctional epoxy resin. The tetrafunctional epoxy resin with the diazaphthone structure is synthesized by virtue of a one-pot two-step method, and an optimized reaction condition is determined through the inspection of the temperature, the time and the material ratio. The synthetic reaction comprises the steps of with diamine monomer with the diazaphthone structure and epoxy chloropropane as the materials, carrying out first-step ring-opening reaction; and dropwise adding a sodium hydroxide solution for ring-closure reaction, and finally, carrying out toluene extraction and washing, so as to obtain a target monomer. Tetraglycidyl-4,4'-diaminodiphenylmethane epoxy resin modified with the tetrafunctional epoxy resin with the diazaphthone structure has very good formation machinability, can be taken as a resin matrix and can be used for preparing high-temperature-resistant and high-performance fiber-reinforced resin-base composite material by virtue of the processes of lamination, mold pressing and RTM.
Owner:DALIAN UNIV OF TECH
Hexa-aza-naphthalene derivative and preparation method and application thereof
ActiveCN112409364ASimple processLow costOrganic chemistryCell electrodesElectrochemical responseOrganosolv
The invention belongs to the field of synthesis of aqueous zinc ion battery electrode materials, and particularly relates to a hexa-aza-naphthalene derivative and a preparation method and applicationthereof. The synthesis method of the hexa-aza-naphthalene derivative comprises the following synthesis steps that 3, 4-diamino-1, 6-phenyl derivative and cyclohexanone octahydrate are added into a reactor under the protection of inert gas and dissolved in an organic solvent; the reaction mixture is stirred continuously in the reflux state, after the reaction is over, heating is stopped, the aceticacid, the deionized water and the ethanol are added for washing, suction filtration is carried out, and the hexa-aza-naphthalene derivative is synthesized. The preparation method of the hexa-aza-naphthalene derivative is simple in process, low in cost, low in energy consumption, good in reproducibility and excellent in performance. The synthesized hexa-aza-naphthalene derivative material not onlycan solve the problem that organic material naphthoquinone derivatives are dissolved in electrolyte, but also is expected to obtain performance with high conductivity so as to guarantee rapid transmission of electrons in the electrochemical reaction process, and has wide application prospects in the field of aqueous zinc ion battery electrode materials.
Owner:CHANGZHOU UNIV
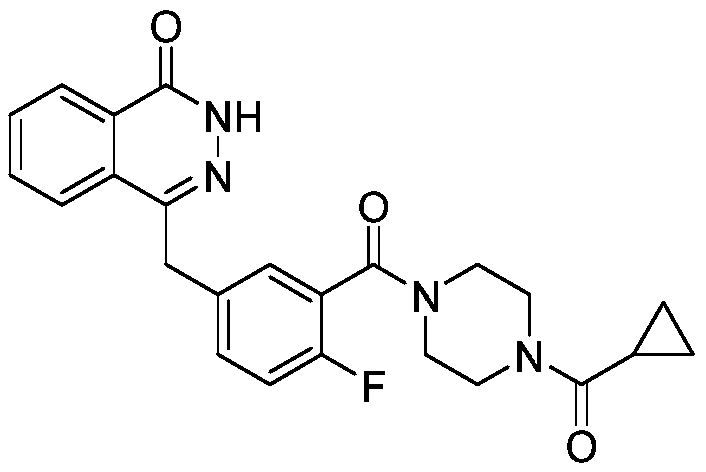
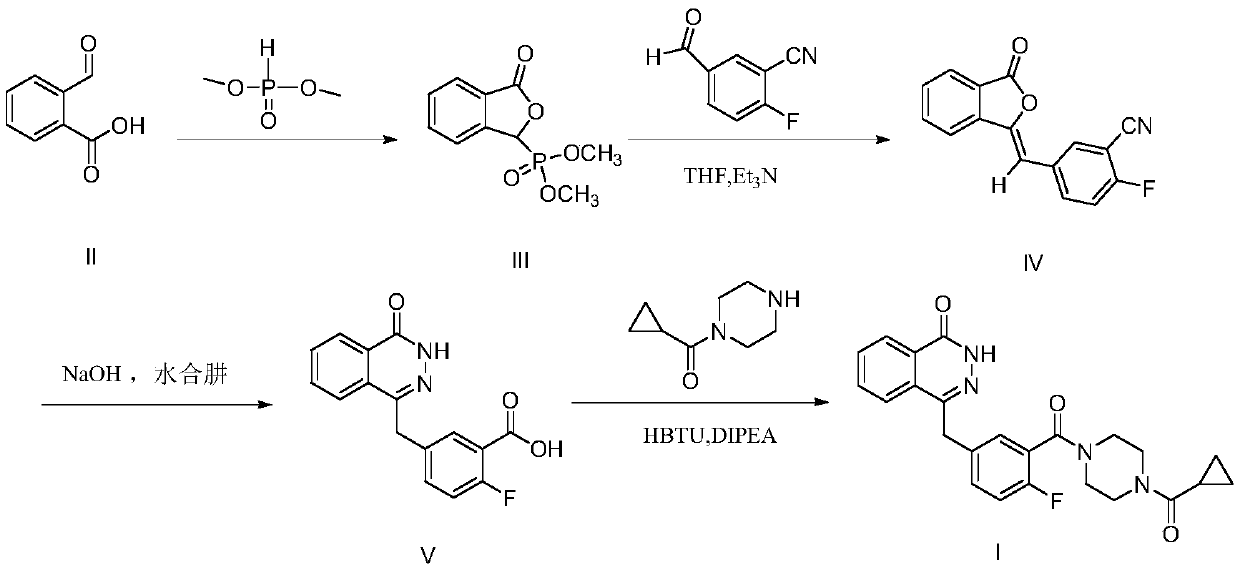
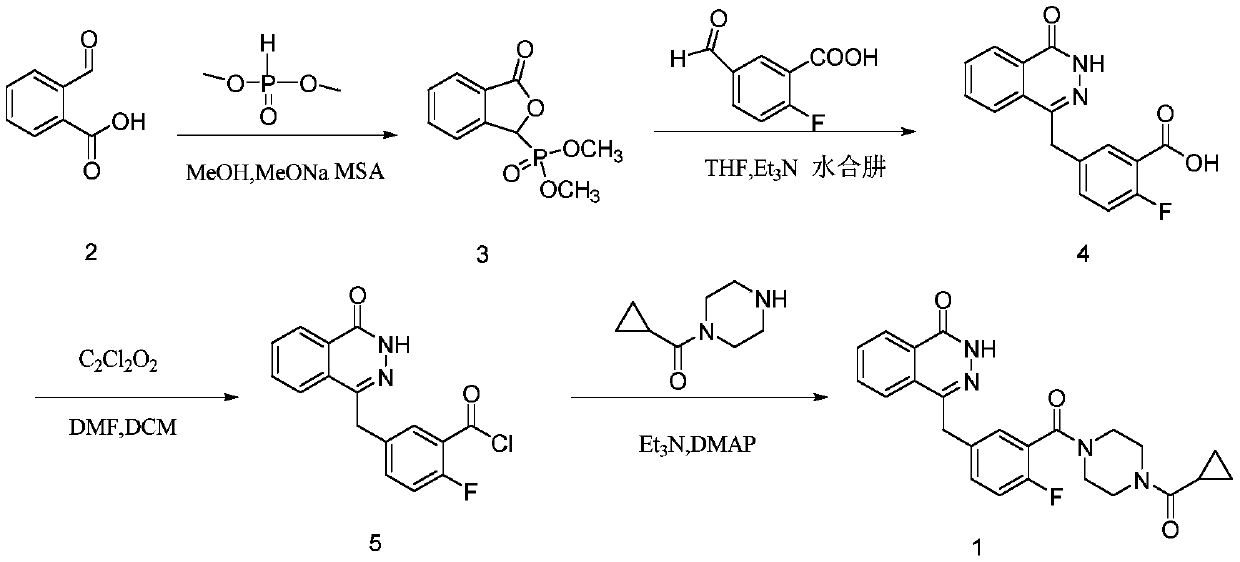
Synthetic method of olaparib
PendingCN110790710ARaw materials are easy to getEasy to handleOrganic chemistryBenzoic acidPhosphorous acid
The invention discloses a synthesis method of olaparib, which comprises the following steps: carrying out reaction on dimethyl phosphite and o-carboxybenzaldehyde to generate (3-oxo-1, 3-dihydroisobenzofuran-1-yl) dimethyl phosphate; enabling (3-oxo-1, 3-dihydroisobenzofuran-1-yl) dimethyl phosphate to react with 2-fluoro-5-formylbenzoic acid to generate 2-fluoro-5-[(4-oxo-3, 4-dihydro naphthyridine-1-yl) methyl] benzoic acid; reacting 2-fluoro-5-[(4-oxo-3, 4-dihydro naphthyridine-1-yl) methyl] benzoic acid with oxalyl chloride to generate 2-fluoro-5-[(4-oxo-3, 4-dihydro naphthyridine-1-yl) methyl] benzoyl chloride; reacting 2-fluoro-5-[(4-oxo-3, 4-dihydro naphthyridine-1-yl) methyl] benzoyl chloride to react with piperazine cyclopropyl ketone, so as to generate olaparib. The invention provides a new olaparib synthesis route, the raw materials are easy to obtain, the operation post-treatment is simple, the reaction conditions of each step are mild, and the total yield reaches 93%.
Owner:SOUTHEAST UNIV
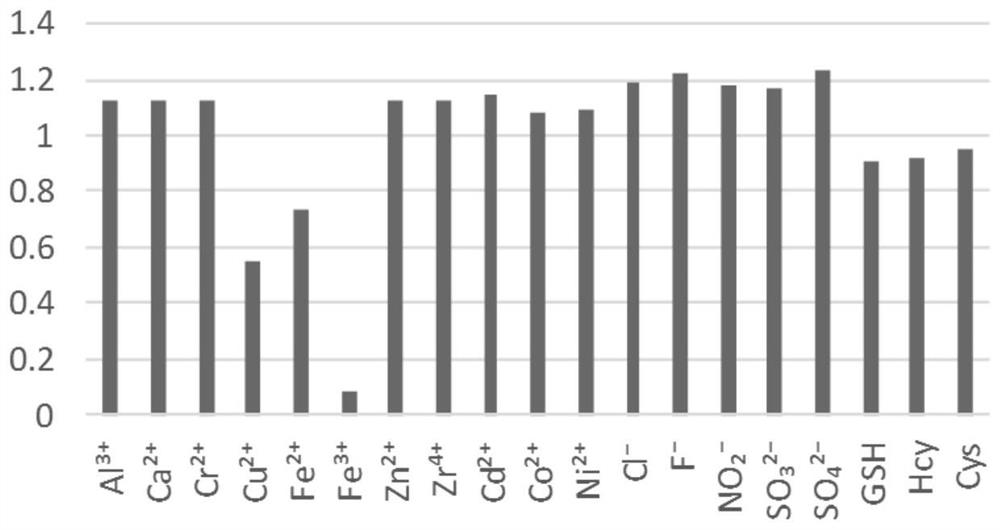
Chromenonaphthyridine-Troger's base Fe<3+> fluorescent probe, and preparation method and application thereof
ActiveCN111909182AMild reaction conditionsShort reaction timeAntibacterial agentsAntimycoticsFluoProbesFluorescence
The invention provides a chromenonaphthyridine-Troger's base Fe<3+> fluorescent probe which has a structural formula as shown in the specification. The chromenonaphthyridine-Troger's base Fe<3+> fluorescent probe is prepared from p-bromoaniline, paraformaldehyde, malononitrile, substituted o-hydroxyacetophenone and the like through a multi-step reaction. A synthesis process of the probe has the advantages of mild reaction conditions, short reaction time and high yield. The chromenonaphthyridine-Troger's base Fe<3+> fluorescent probe emits blue fluorescence in a solution and green fluorescencein a solid state; fluorescence brightness is high; a part of compounds have specific recognition capability on Fe<3+>, and are low in detection limit, quick in response and good in stability; and a part of the compounds have a good inhibition effect on bacillus subtilis.
Owner:XUZHOU NORMAL UNIVERSITY
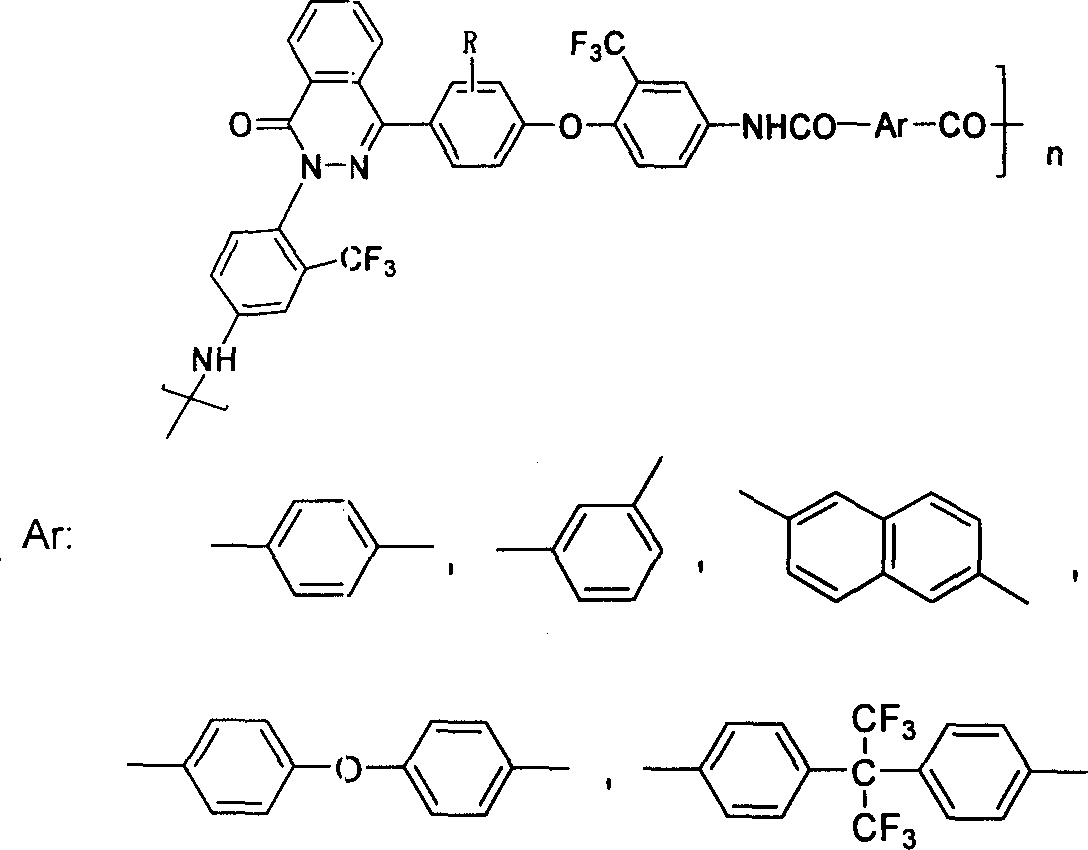
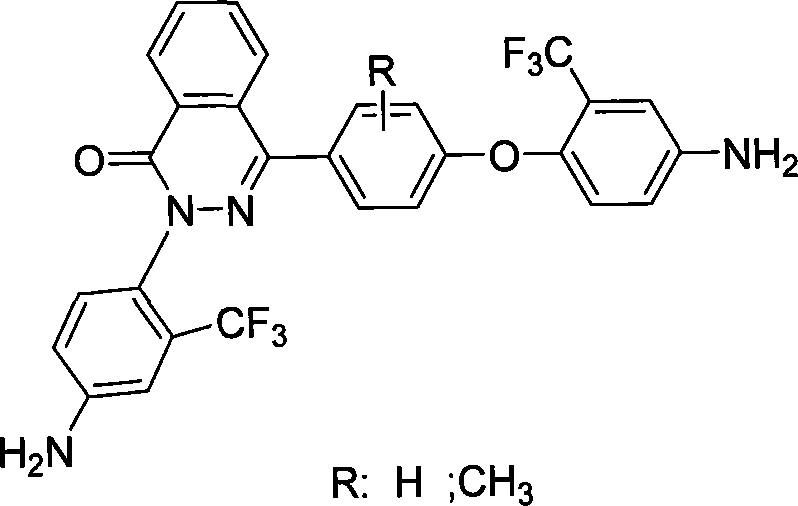
Aromatic polyamide containing fluorine and diamine monomer containing fluorine based on naphthyridine ketone structure and method of producing the same
The present invention discloses a poly aromatic amide compound containing fluorine and based on the structure of di-nitrone phthalazinone, a diamine monomer containing fluorine and the preparation method. The bisphenol type monomer with the structure of phthalazinone and the 2-chlorine-5-nitro toluene trifluoromethyl react to produce a dinitro compound through nucleophilic substitution; the dinitro compound is reduced to be diamine monomer containing fluorine. The poly aromatic amide uses diamine monomer containing fluorine and diacid for condensation and polymerization to get novel poly aromatic amide of good solubility; the specific viscosity is between 0.41dL / g to 0.87dL / g; the conversion rate of glass is above 302 DEG C; the decomposition temperature is above 437 DEG C. The high-molecule compound of the poly aromatic amide compound containing fluorine can be used for advanced technological fields such as aerospace, high-precision electronics and information industry and so on.
Owner:HUAQIAO UNIVERSITY
Polyarylene sulfide sulfone containing phthalazinone structure and preparation method thereof
PendingCN111548495AHigh glass transition temperatureHigh thermal decomposition temperaturePolymer sciencePtru catalyst
The invention discloses polyarylene sulfide sulfone containing a phthalazinone structure and a preparation method thereof. The preparation method comprises the following steps: taking dihalogen diphenyl sulfone and an equimolar bisphenol-like monomer containing a phthalazinone structure as raw materials; then in a polar solvent or an aprotic polar solvent, with alkali metal as a catalyst, carryingout heating dehydration in a nitrogen atmosphere to remove moisture and an azeotropic dehydrating agent; performing polymerization, cooling the reaction product, diluting the reaction product, precipitating the reaction product in a precipitator, and filtering and drying the mixture to obtain the polyarylene sulfide sulfone. The polymer is designable in structure and has excellent flexibility andductility. Meanwhile, the polymer has good solubility, excellent thermal stability and good processability, so that the series of polymers are expected to be applied to the field of polymer flame retardance.
Owner:SHANGHAI INST OF TECH
1,8-naphthyridine compound and organic light-emitting device using the same
InactiveUS7833634B2Increase brightnessIncreased durabilityOrganic chemistryDischarge tube luminescnet screensArylOrganic light emitting device
Provided a novel 1,8-naphthyridine compound represented by the following general formula [I]:wherein R1 to R6 each represent a hydrogen atom; an alkyl group, a halogen atom; a trifluoromethyl group; and a cyano group, and may be the same as or different from one another, and that at least two of R1 to R6 each represent an aralkyl group, an aryl group, a heterocyclic group, a condensed polycyclic aromatic group, a condensed polycyclic heterocyclic group and an aryloxy group which may be substituted; and a substituted amino group. The 1,8-naphthyridine is employed in an organic compound layer provided between a pair of electrodes in an organic light-emitting device.
Owner:CANON KK
Preparation method of ethyl benzoylacetate
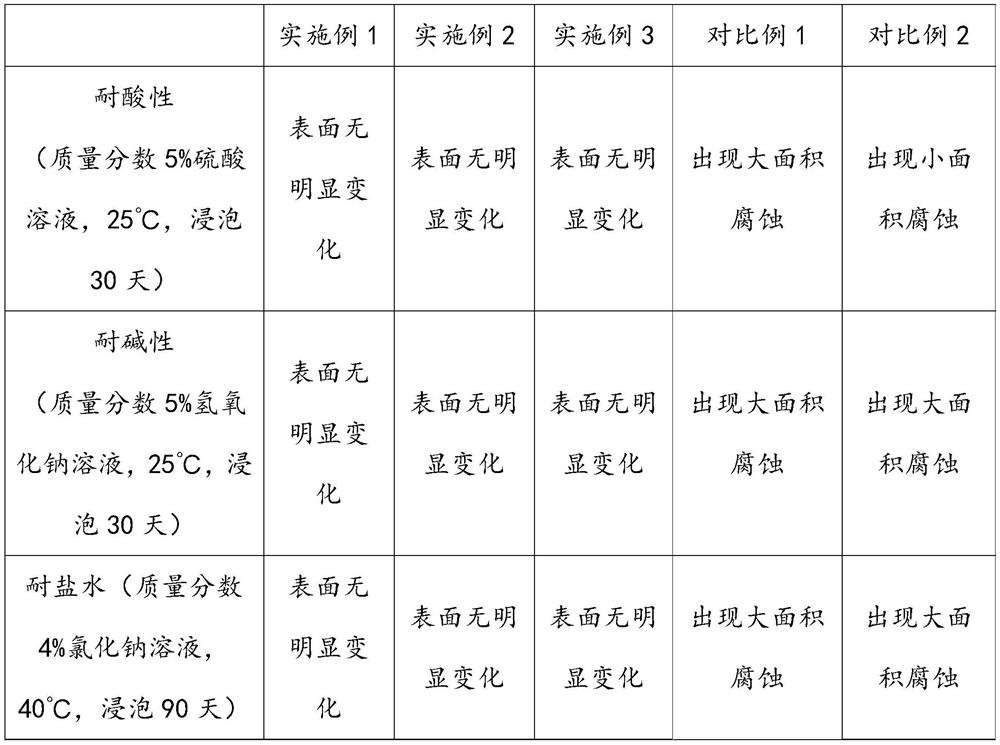
ActiveCN113200855AImprove corrosion resistanceNo corrosionSemi-permeable membranesOrganic compound preparationEpoxySodium bicarbonate
The invention discloses a preparation method of ethyl benzoylacetate, and belongs to the technical field of chemical synthesis. The preparation method comprises the following steps of: adding sodium bicarbonate into carbon tetrachloride, slowly adding ethyl acetoacetate, dropwise adding benzoyl chloride for reaction, adding sodium hydroxide for reaction, and filtering after the reaction is finished to obtain ethyl benzoylacetate. A microporous filter membrane is used in the process of preparing ethyl benzoylacetate. A reinforcing filler is prepared in the process of preparing the microporous filter membrane, and nano silicon dioxide and aniline are treated by the reinforcing filler, so that polyaniline is grafted on the surface of the nano silicon dioxide. Then, the nano silicon dioxide and modified graphene are subjected to ultrasonic treatment to obtain a reinforced substrate. 4-(4-hydroxyphenyl)-2, 3-phthalazine-1-one and epichlorohydrin are subjected to a reaction to prepare epoxy resin, then the epoxy resin and amino groups on the reinforced substrate are cured to prepare a reinforcing filler, the reinforcing filler can enhance the corrosion resistance of the microporous filter membrane, and the microporous filter membrane cannot be corroded after being used for a long time.
Owner:江苏巨莱生物医药有限公司
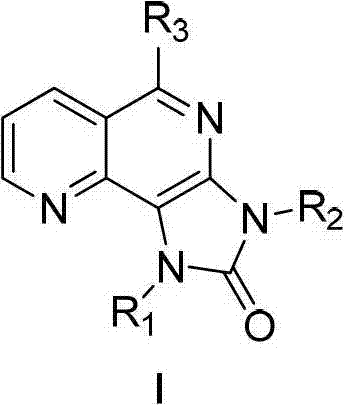
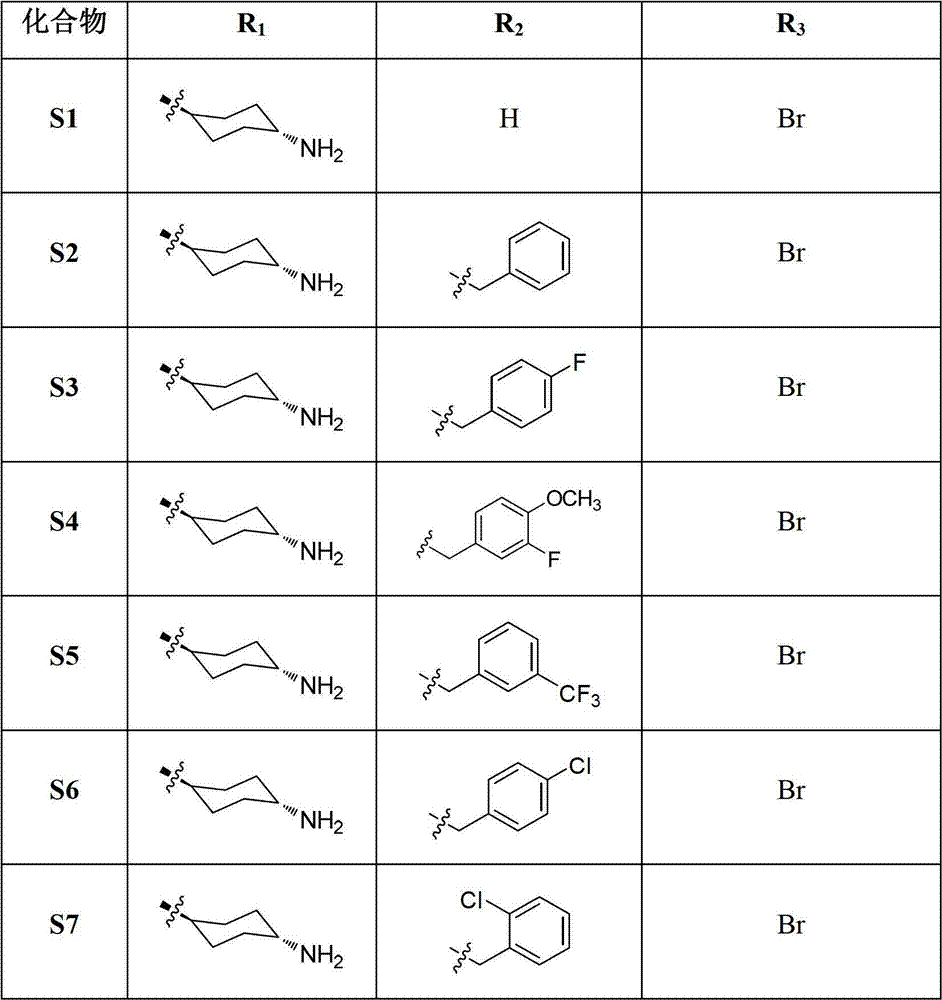
Tri-substituted imidazo diazanaphthalene ketone compounds, preparation method thereof and applications thereof
The invention relates to 1,3,5-tri-substituted-1H-imidazo[4,5-h]1,6-diazanaphthalene-2(3H)-one compounds shown as the general formula I, isomers thereof and pharmaceutically acceptable salts thereof, a preparation method and applications thereof, and compositions comprising the compounds. The invention also relates to applications of the compounds, the isomers thereof, the pharmaceutically acceptable salts thereof and the compositions comprising the compounds, as protein multi-target kinase inhibitors, in preparation of medicines used for treating protein kinase related disease, especially c-Met related disease, such as neoplastic disease.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Compound, electroluminescent device and application of device
PendingCN113461627AImprove performanceReduce the driving voltageIndium organic compoundsSolid-state devicesHost materialStructural unit
The invention provides a compound, an electroluminescent device and application of the device. The compound has a structure as shown in a formula I, is a novel triarylamine compound which is connected with an aza-aromatic ring and contains a naphthalene or aza-naphthalene structural unit, and can be used as a charge transport material or a host material in the electroluminescent device. According to the compound, the structure is improved through the design of a molecular structure and substituent groups, the driving voltage of an electroluminescent device can be reduced, the luminous efficiency is improved, the service life of the device is remarkably prolonged, and the electroluminescent device can fully meet the requirements of commercial application.
Owner:BEIJING SUMMER SPROUT TECH CO LTD
Efficient boat bottom antibacterial coating as well as preparation method and application methods thereof
InactiveCN106118392ASpeed up the drying processReasonable compositionAntifouling/underwater paintsPaints with biocidesXylyleneMethyl palmoxirate
The invention provides efficient boat bottom antibacterial coating as well as preparation method and application methods of the efficient boat bottom antibacterial coating. The efficient boat bottom antibacterial coating provided by the invention is prepared from polyester resin, isophthalic acid, talcum powder, lithopone, 1-methyl-1,4 dihydroquinoline, iron blue, barium sulfate, calcium carbonate, copper chloride, rosin, xylol and an addition agent. The efficient boat bottom antibacterial coating provided by the invention has the advantages of reasonable components, special antibacterial property of the product, high stability and good effect. The preparation method of the efficient boat bottom antibacterial coating is simple and feasible in process, high in production efficiency, low in utilization cost, simple to operate and easy to realize; the produced product is stable in quality, convenient to use and good in adaptability, and has relatively high popularization and application value.
Owner:SUZHOU TIANJIANHENG ELECTRONICS INFORMATION TECH CO LTD
Acenaphtho-aza naphthalene derivative, preparation method thereof, infrared electronic device and infrared device
ActiveCN111362951ASpecial fused ring structureImprove thermal stabilityOrganic chemistrySolid-state devicesOrganic solar cellQuantum yield
The invention provides an acenaphtho-aza naphthalene derivative, a preparation method thereof, an infrared electronic device and an infrared device, and relates to the technical field of organic photoelectric materials. By introducing the condensed ring structure of the acenaphtho-aza naphthalene derivative, the obtained acenaphtho-aza naphthalene derivative is excellent in film-forming property and thermal stability and relatively high in fluorescence quantum yield, and can be used to prepare an organic light-emitting device, an organic field effect transistor and an organic solar cell. Furthermore, the acenaphtho-aza naphthalene derivative can be used as a constituent material of a hole injection layer, a hole transport layer, a light emitting layer, an electron blocking layer, a hole blocking layer or an electron transport layer, and can reduce a driving voltage, improve efficiency, brightness, and lifespan, and the like. In addition, the preparation method of the acenaphtho-aza naphthalene derivative is simple, raw materials are easy to obtain, and industrial development requirements can be met.
Owner:SUZHOU JOYSUN ADVANCED MATERIALS CO LTD
Titanium dioxide/polyhexaaza-naphthalene triphenylamine core-shell structure composite film as well as preparation method and application of titanium dioxide/polyhexaaza-naphthalene triphenylamine core-shell structure composite film
The invention discloses a titanium dioxide / polyhexaazanaphthalene triphenylamine core-shell structure composite film as well as a preparation method and application of the titanium dioxide / polyhexaazanaphthalene triphenylamine core-shell structure composite film. The method comprises the following steps: mixing deionized water and 37 wt% concentrated hydrochloric acid, adding tetrabutyl titanate,dipping fluorine-doped tin oxide conductive glass into the mixed solution, putting the mixed solution into a stainless steel reaction kettle, putting the stainless steel reaction kettle into an electric oven at 120-180 DEG C, and completely reacting to obtain a fluorine-doped tin oxide conductive glass / titanium dioxide electrode; in a three-electrode electrolytic tank system, taking the fluorine-doped tin oxide conductive glass / titanium dioxide electrode as a working electrode, a gold electrode or a platinum electrode as an auxiliary electrode and a silver / silver chloride electrode as a reference electrode, carrying out an electrochemical polymerization reaction at room temperature under the condition of a voltage of -2V to 2V by adopting a cyclic voltammetry to obtain the titanium dioxide / polyhexaazanaphthalene triphenylamine core-shell structure composite film. The preparation method disclosed by the invention is relatively low in cost, simple to operate, green and environment-friendly, and the composite film has excellent electrochromic performance.
Owner:ZHEJIANG UNIV OF TECH
1,4-di(para hydroxybenzene) based-2,3-quinazoline and synthesis method thereof
The invention discloses 1,4-di(para hydroxybenzene) based-2,3-quinazoline and a synthesis method thereof. In the synthesis method, a Grignard reagent is prepared from para-halogen anisole and magnesium ribbons and is reacted with phthalic anhydride in a tetrahydrofuran / toluene, 2-methyl tetrahydrofuran / toluene, or diethyl ether / toluene mixing solvent to obtain benzol-1,2-di(para-methoxyl)benzophenone; under the solvent of glacial acetic acid or alcohol, the benzol-1,2-bi(para-methoxyl)benzophenone is reacted again with hydrazine hydrate to obtain 2,3-quinazoline1,4-bi-hydroxybenzene; and finally the 2,3-quinazoline1,4-bi-hydroxybenzene is demethylated under the participation of 40 percent of hydrobromic acid by taking the glacial acetic acid as the solvent to obtain the product. The invention provides a new monomer for developing a high-property polymer material with a quinazoline structure. The invention has reasonable and simple selection for a technical route as well as convenience and easy operation and provides a successful synthesis method for the synthesis of the intermediate of the high polymer product technically.
Owner:DALIAN JOIN KING FINE CHEM CO LTD
Method for preparing phthalonitrile resin cured product by amino-terminated polyarylether curing agent containing phthalazinone biphenyl structure
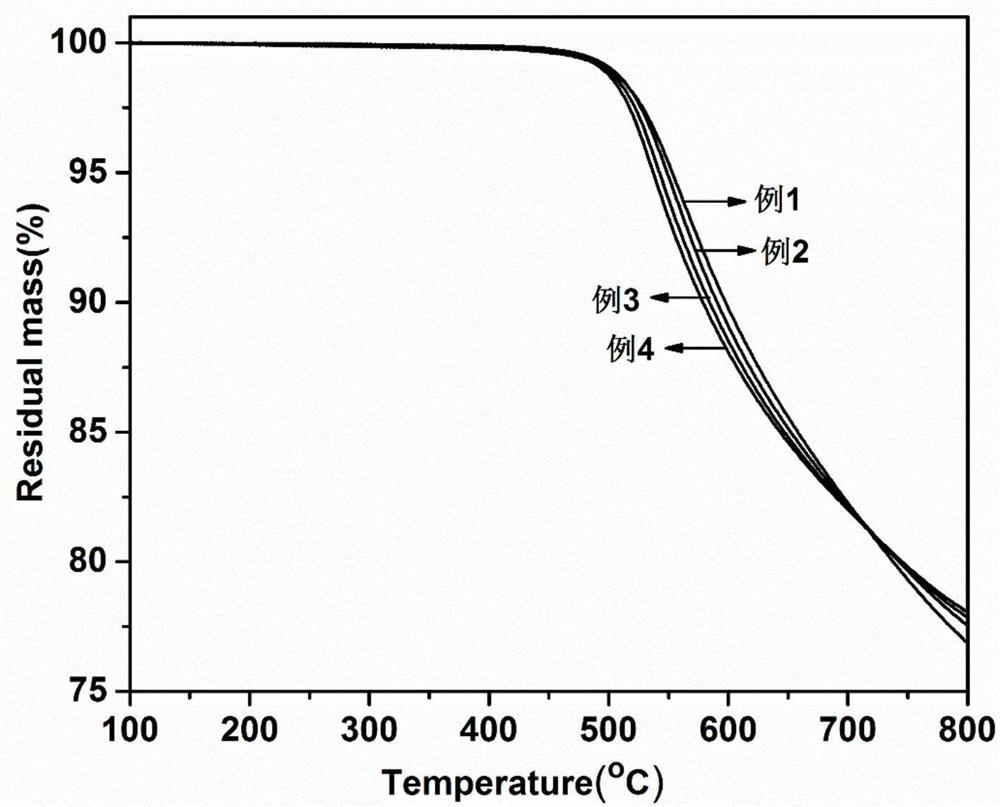
The invention provides a method for preparing a cured phthalonitrile resin with an amino-terminated polyaryl ether curing agent containing a phthalazinone biphenyl structure, and belongs to the field of material science and technology. The amino-terminated polyaryl ether containing phthalazinone biphenyl structure is used as the curing agent to mix with the phthalonitrile resin precursor, and the temperature is programmed to be cured. The amino-terminated polyarylether curing agent containing phthalazinone biphenyl structure that the present invention adopts has excellent thermal stability, and the cured product of resorcinol-based phthalonitrile resin prepared with it as curing agent is heated in nitrogen The maximum thermal weight loss temperature of 5% in the atmosphere can reach 553.2°C, the maximum carbon residue rate at 800°C is 78.1%, and the glass transition temperature is higher than 400°C; the lowest melt viscosity is 0.167Pa·s, showing excellent melt Processing fluidity; has a very wide processing window, the widest can reach 194.6 ℃. The cured phthalonitrile resin has excellent thermal stability and thermomechanical properties.
Owner:DALIAN UNIV OF TECH
Thermally activated delayed fluorescent material and preparation method thereof
InactiveCN113321656ATransient fluorescence lifetime delayLong fluorescence lifetimeOrganic chemistryLuminescent compositionsDisplay deviceAniline
The invention belongs to the technical field of photoelectric display devices, and particularly relates to a thermally activated delayed fluorescent material and a preparation method thereof. The structural formula of the thermally activated delayed fluorescent material is shown as a formula (I). The invention also provides the preparation method of the thermally activated delayed fluorescent material. The preparation method comprises the following step: reacting N, N-diphenyl-4-(4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborane-2-yl)aniline with 2, 5, 8-tribromo-1, 3, 3a1, 4, 6, 7, 9-heptaazanaphthalene to obtain the compound as shown in the formula (I). The invention provides the thermally activated delayed fluorescence material and the preparation method thereof to solve the technical problems that the efficiency of the existing TADF micromolecule blue light material and the electroluminescent device thereof is not high, and the aggregation quenching luminescence effect is easy to generate.
Owner:广州万物物联科技有限公司
Small-molecule luminescent materials based on 1,3-benzodiazine (quinazoline) and production method and application of small-molecule luminescent materials based on 1,3-benzodiazine (quinazoline)
InactiveCN110724128AImprove luminous efficiencyEasy to synthesizeOrganic chemistrySolid-state devicesArylBenzodiazine
The invention provides small-molecule luminescent materials based on 1,3-benzodiazine (quinazoline) and a production method and application of the small-molecule luminescent materials based on the 1,3-benzodiazine (quinazoline), and belongs to the technical field of organic photoelectric materials. A structural formula of the luminescent materials is shown in a formula (1)as shown in the description, in theformula (1), each of R1, R2, R3, R4, R5 and R6 is selected from one of a hydrogen atom, an alkoxy group, an alkylthio group, an alkylamine group, an arylamine group, an aryloxy group, an arylthio group, an aryl group and an aromatic heterocyclic group, and at least one of R1, R2, R3, R4, R5 and R6 is an aromatic heterocyclic group. According to the small-molecule luminescent materials based on the 1,3-benzodiazine (quinazoline) and the production method and application of the small-molecule luminescent materials based on the 1,3-benzodiazine (quinazoline), the 1,3-benzodiazine (quinazoline) is introduced into TADF materials for the first time, and high luminescence efficiency is achieved. The materials are simple to synthesize, steps are short, and synthesis cycles are short. Reaction yields are high, and the yields of all the steps are higher than 70%, so that the cost is saved. The OLED luminescent efficiency is high, and the highest efficiency can exceed 20%.
Owner:HARBIN INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com