Hexa-aza-naphthalene derivative and preparation method and application thereof
A technology of hexaaza and derivatives, which is applied in the field of synthesis of electrode materials for water-based zinc-ion batteries, can solve the problems of high price, and achieve the effects of low cost, good reproducibility and high cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
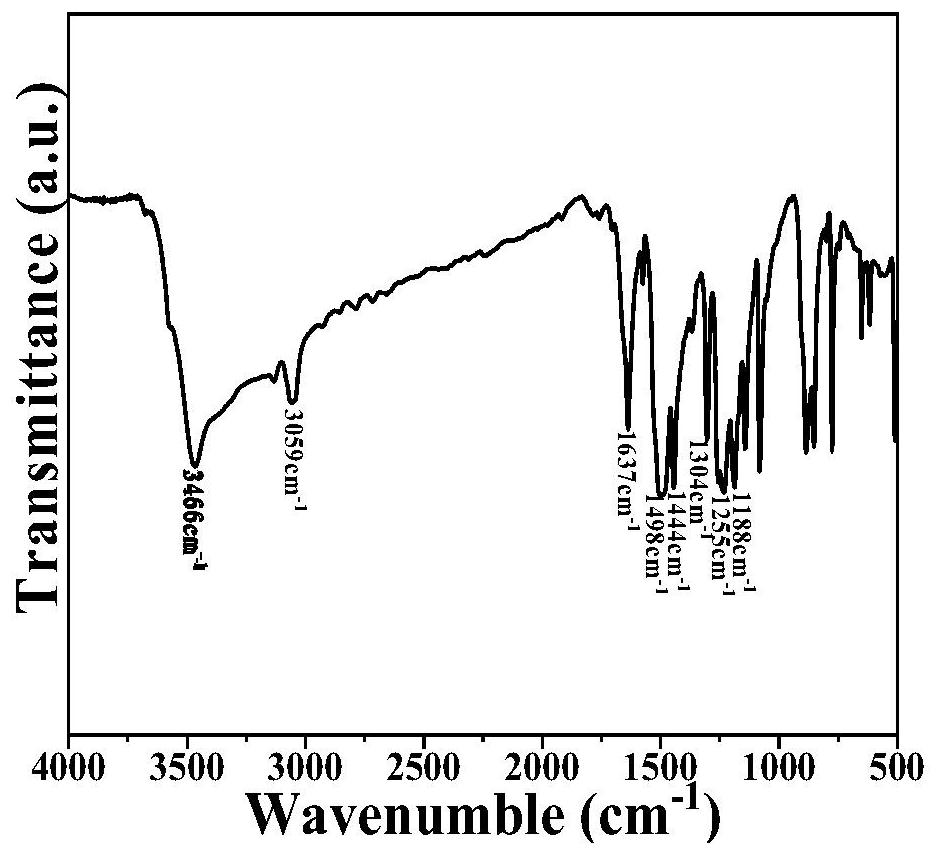
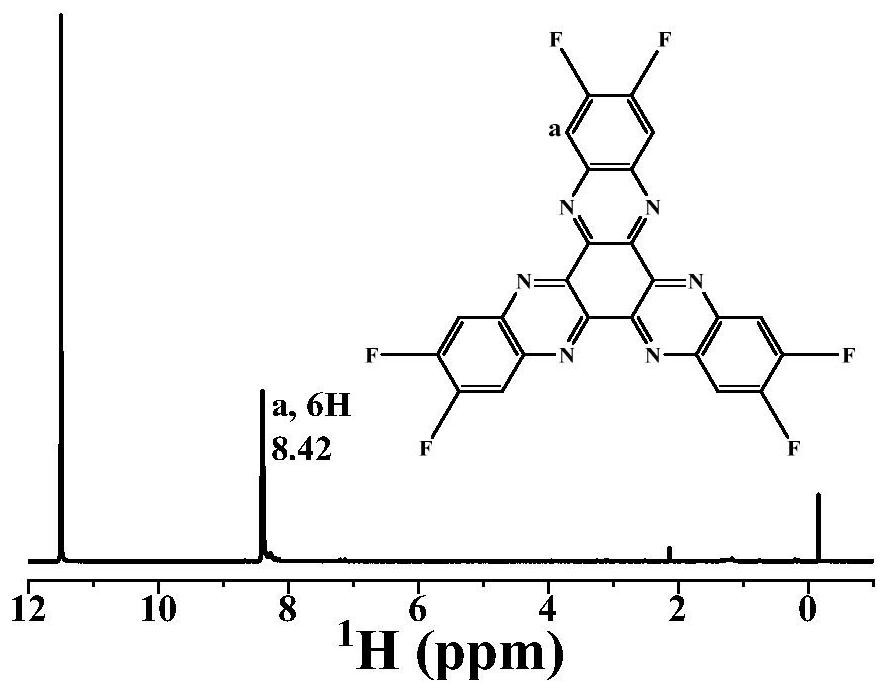
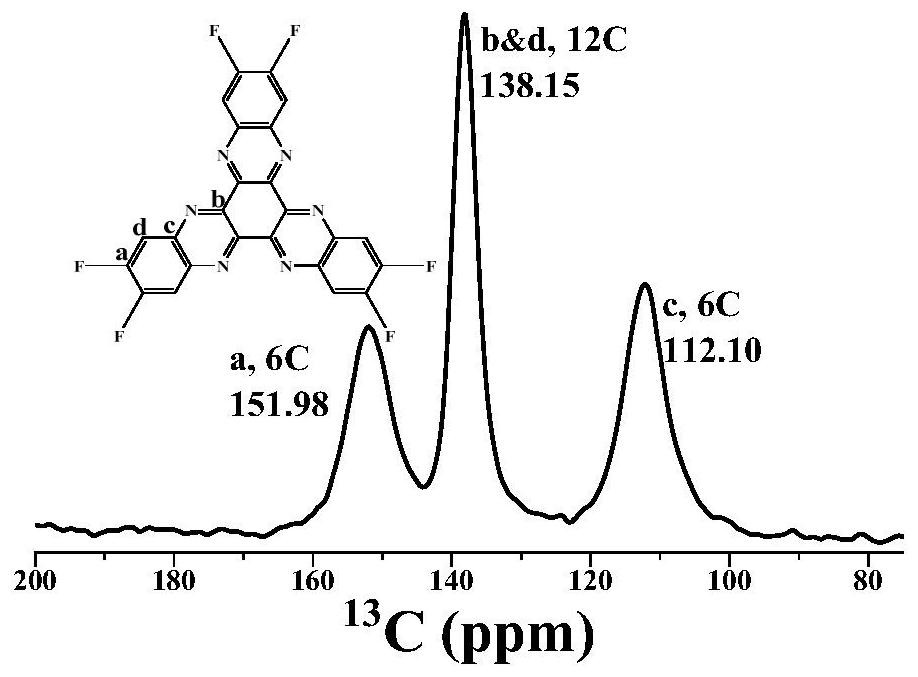
[0077]In a 100mL three-necked flask was added 60mL of acetic acid, while adding 4,5-difluoro-1,2-phenylenediamine (0.690g, 4.8mmol), cyclohexanone octahydrate (0.499g, 1.6mmol), under nitrogen Under the protection, the reaction mixture was kept stirring under reflux. The reaction time was 24 hours. After the reaction, the heating was stopped, and acetic acid, deionized water and ethanol were added to wash several times. The solid was collected by suction filtration and dried under vacuum at 100°C for 6 hours to obtain the compound. A: 2,3,8,9,14,15-hexafluorohexaazanaphthalene, the yield was 84.3%.figure 1 Is the infrared spectrum,figure 2 Is the NMR spectrum,image 3 Is a solid nuclear magnetic carbon spectrum,Figure 4 It is a mass spectrum, which is used as the active material of the electrode material. According to the mass ratio of the active material: Ketjen Black: Binder (PVDF) = 30%: 60%: 10%, mix and grind, and then add the diffusing agent (NMP) dropwise. Grinding, coating on...
Embodiment 2
[0083]In a 100mL three-necked flask, add 30mL of acetic acid and 30mL of ethanol, and add 4,5-dichloro-o-phenylenediamine (0.85g, 4.8mmol), cyclohexanone octahydrate (0.499g, 1.6mmol) at the same time, under nitrogen protection The reaction mixture was continuously stirred under reflux status. The reaction time was 24 hours. After the reaction, the heating was stopped, and acetic acid, deionized water, and ethanol were added to wash several times. The solid was collected by suction filtration, and then the product was transferred to the flask and added 30% nitric acid (50 mL) was stirred and refluxed at 140°C for 3 hours. The solid was collected by filtration, the filter cake was thoroughly washed with deionized water and ethanol in sequence, and dried in vacuum to obtain compound B: 2,3,8,9,14,15-hexachlorohexaazin with a yield of 87%.Figure 22 Is the rate stability diagram of button battery;Figure 23 It is the charging and discharging curve diagram of the button cell under differe...
Embodiment 3
[0085]The experimental method is the same as in Example 1, except that 4,5-difluoro-1,2-phenylenediamine is added to the mass of reactants (1.380g, 9.6mmol) to obtain compound C: 1,2,3,4,7,8 ,9,10,13,14,15,16-Dodecafluorohexaazanaphthalene, the yield was 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com