Acenaphtho-aza naphthalene derivative, preparation method thereof, infrared electronic device and infrared device
An electronic device, azanaphthalene technology, applied in the field of organic photoelectric materials, can solve problems such as external quantum efficiency attenuation, and achieve the effects of strong electron pulling ability, good external quantum efficiency, and reduced driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
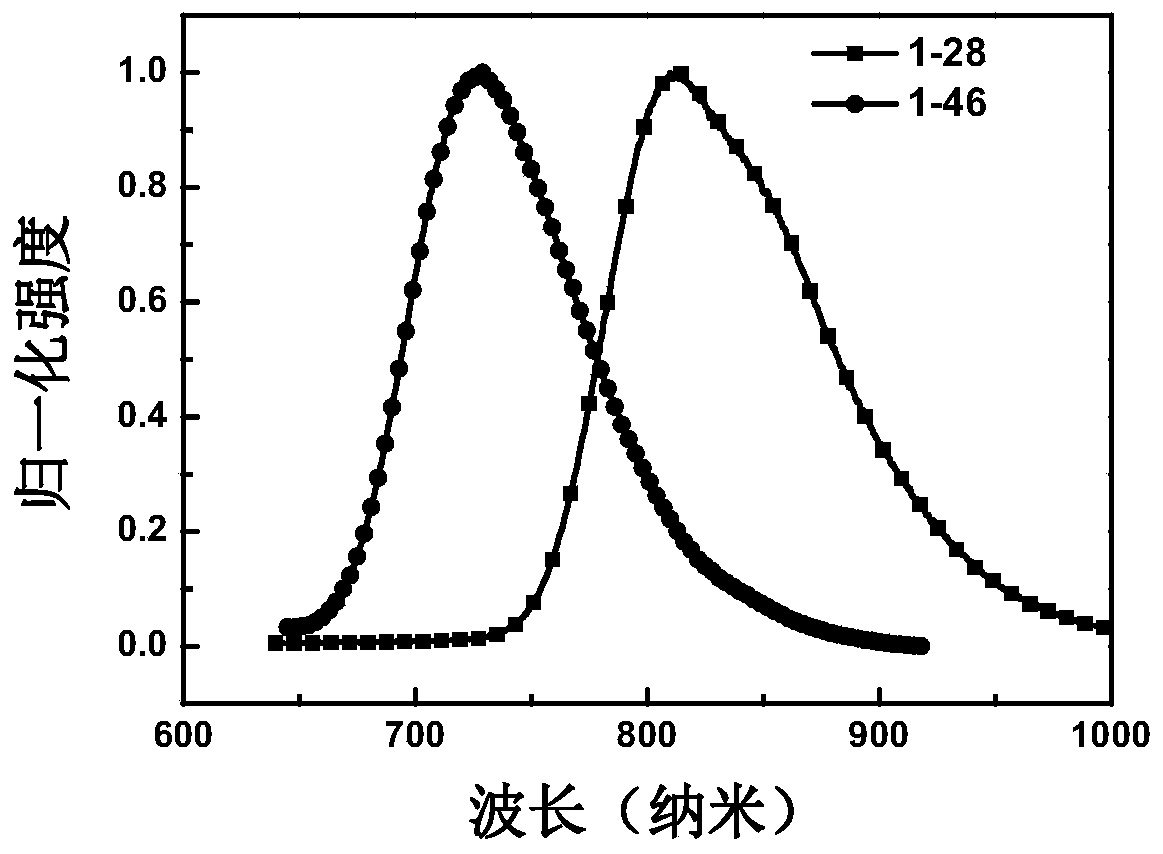
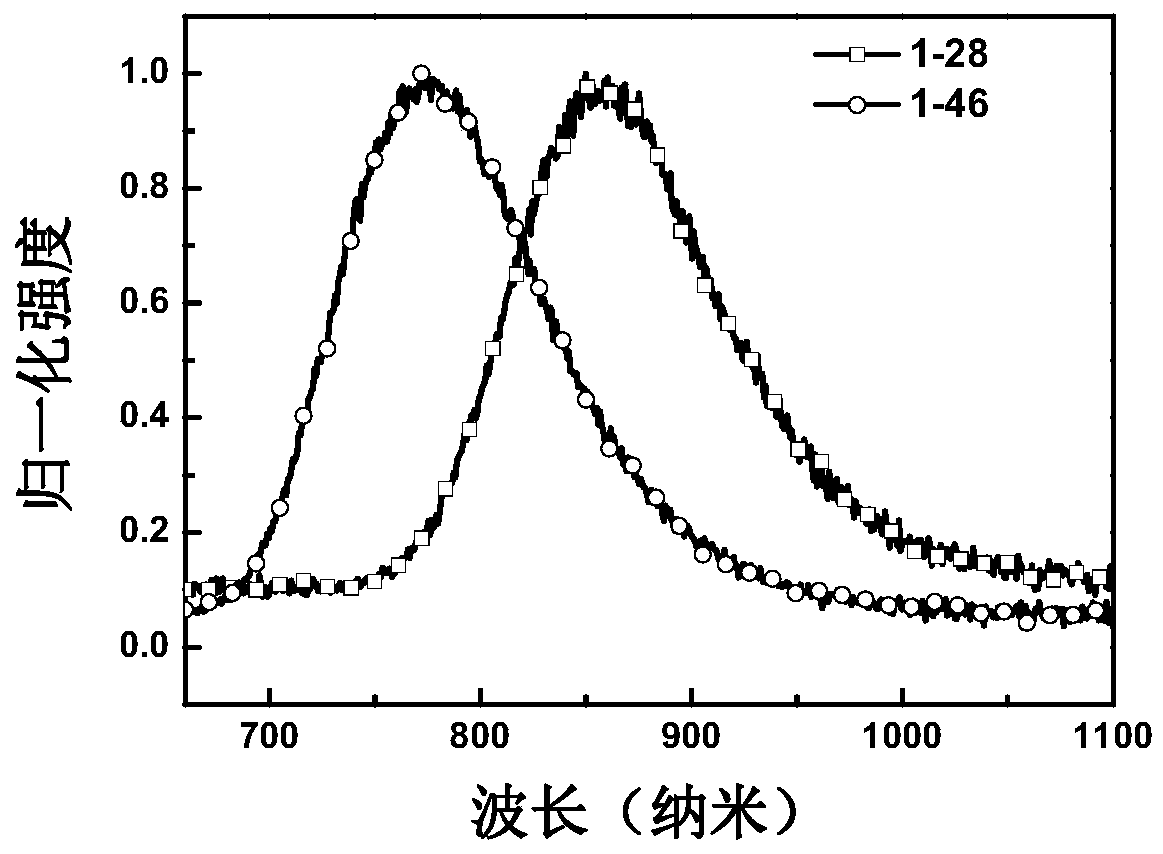
[0135] Embodiment 1: the synthesis of compound 1-28
[0136] (Synthesis of intermediate M1)
[0137] The synthetic route of intermediate M1 is as follows:
[0138]
[0139] Add 7.0g (30mmol) 5,6-dicyanoacenaphthylene-1,2-dione, 5.7g (30mmol) 5-bromo-2,3-diaminopyrazine and 100mL glacial acetic acid in a clean 250mL single-necked bottle , gradually warmed to reflux and reacted overnight under reflux. After the reaction was completed, the heating was stopped, and the system cooled down by itself. The reaction solution was poured into 1L of ice water, collected by suction filtration, squeezed and dried, and further purified by column chromatography (350 mesh silica gel, eluent: petroleum ether: dichloromethane = 2:3 (V / V)) Afterwards, 9.4 g of yellow solid was obtained, and the yield was 82%. MS (EI): m / z: 383.92 [M + ]. Anal.calcd for C 18 h 5 BrN 6 (%): C 56.13, H 1.31; found: C 56.01, H 1.26.
[0140] (Synthesis of Compound 1-28)
[0141] The synthetic route of c...
Embodiment 2
[0144] Embodiment 2: the synthesis of compound 1-46
[0145] (Synthesis of compound 1-46)
[0146] The synthetic route of compound 1-46 is as follows:
[0147]
[0148] Under nitrogen, 13.8g (47.8mmol) N-phenyl-3-carbazole boronic acid, 8.4g (79.6mmol) anhydrous sodium carbonate, 15.3g (39.8mmol) M1, 470.8 mg (4.8 mmol) of tetrakis(triphenylphosphine palladium) and 100 mL of mixed solvent (toluene:water:ethanol=5:1:1 (V / V)). The system was gradually heated to reflux and reacted overnight under reflux. After the reaction was completed, the heating was stopped, and the reaction system was cooled to room temperature by itself. The reaction solution was poured into about 200 mL of water, and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and further purified by column chromatography (350 mesh silica gel, eluent: petroleum ether: dichloromethane = 3:2 (V / V)) to obtain a red solid 18.3 g, yield ...
Embodiment 3
[0149] Embodiment 3: the synthesis of compound 1-54
[0150] (Synthesis of Intermediate M2)
[0151] The synthetic route of intermediate M2 is as follows:
[0152]
[0153] Add 7.0g (30mmol) 5,6-dicyanoacenaphthylene-1,2-dione, 5.3g (30mmol) 4.5-dichloro-2.3-diaminopyrazine and 100mL glacial acetic acid in a clean 250mL single-necked bottle, Gradually raise the temperature to reflux and react overnight under reflux. After the reaction was completed, the heating was stopped, and the system cooled down by itself. The reaction solution was poured into 1L of ice water, collected by suction filtration, squeezed and dried, and further purified by column chromatography (350 mesh silica gel, eluent: petroleum ether: dichloromethane = 2:3 (V / V)) Afterwards, 9.2 g of yellow solid was obtained, and the yield was 82%. MS (EI): m / z: 374.32 [M + ]. Anal.calcd for C 18 h 4 Cl 2 N 6 (%): C 57.63, H 1.07; found: C 57.51, H 1.05.
[0154] (Synthesis of compound M3)
[0155] The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com