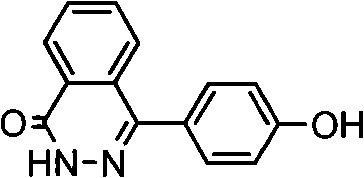
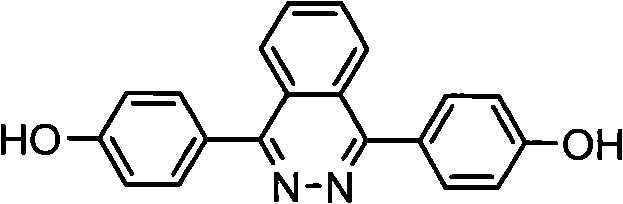
1,4-di(para hydroxybenzene) based-2,3-quinazoline and synthesis method thereof
A technology of naphthyridine and p-hydroxybenzene, which is applied in the field of heterocyclic compounds in organic chemistry, can solve the problem of no literature report on the synthesis method, and achieve the effect of easy operation and reasonable selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
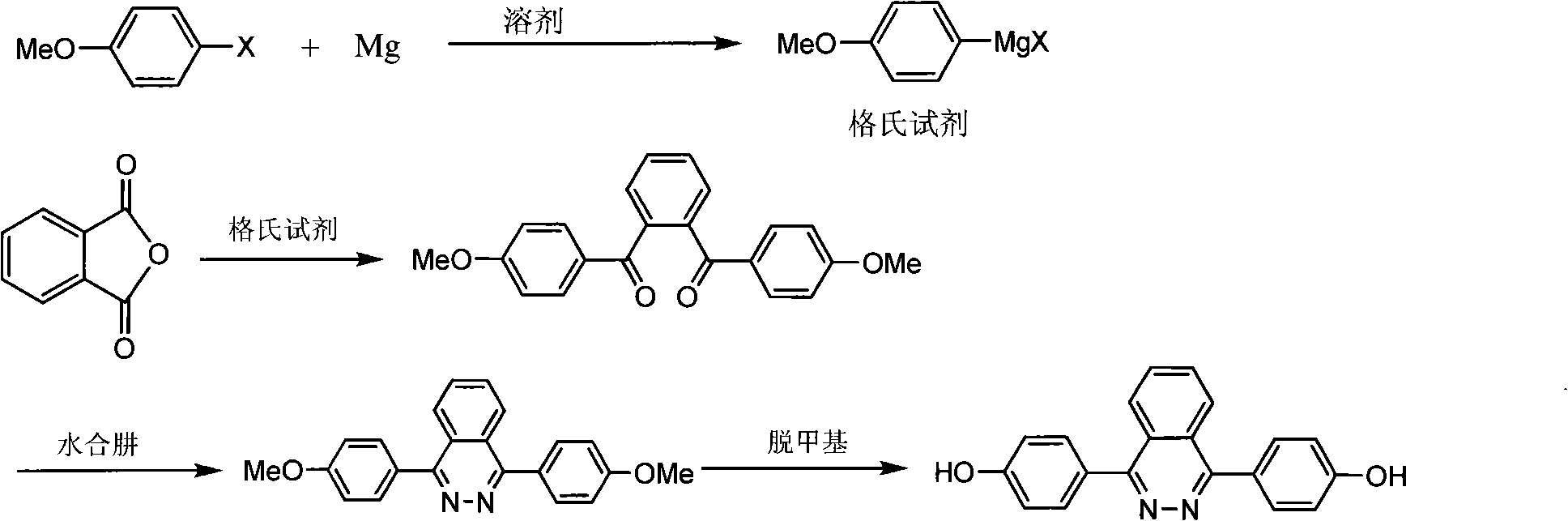
[0020] 1. Preparation of Grignard reagent:
[0021] (1) Add 9.72 grams (0.40 moles) of magnesium chips, 110 grams of dried tetrahydrofuran, and 100 grams of toluene into a four-neck flask with a mechanical stirrer, a thermometer, an addition funnel, and a condenser, and start stirring. Weigh 42.2 g (0.20 mol) of p-bromoanisole into the addition funnel and set aside.
[0022] (2) The temperature in the bottle is 30° C., and 15% of the total mass of p-bromoanisole is added dropwise to the reaction bottle to initiate the reaction, and the temperature rises.
[0023] (3) Continue to add p-bromoanisole dropwise, the temperature is controlled at 30-40°C, after the dropwise addition is completed, continue to stir at 30-40°C for 3h, and the reaction of the raw materials is completed.
[0024] 2. Preparation of intermediate diketone:
[0025] Dissolve 9.9 grams (0.067 moles) of phthalic anhydride in the mixed solvent tetrahydrofuran and toluene (70 grams and 30 grams of tetrahydrofur...
Embodiment 1-2
[0031] Similar reaction with embodiment 1-1. In the reaction step 1, the raw material is p-chloroanisole instead of p-bromoanisole, and the consumption is also 0.20 moles. All the reactions were the same as those in Example 1-1. The obtained solid was dried and weighed 7.45 grams. The purity by HPLC analysis was 98.9%, and the yield was 70.5%.
Embodiment 1-3
[0033] Similar reaction with embodiment 1-1. In the reaction step 1, the raw material is p-iodoanisole instead of p-bromoanisole, and the consumption is also 0.20 moles. All the reactions were the same as those in Example 1-1. The obtained solid was dried and weighed 7.43 grams. The purity by HPLC analysis was 99%, and the yield was 70.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com