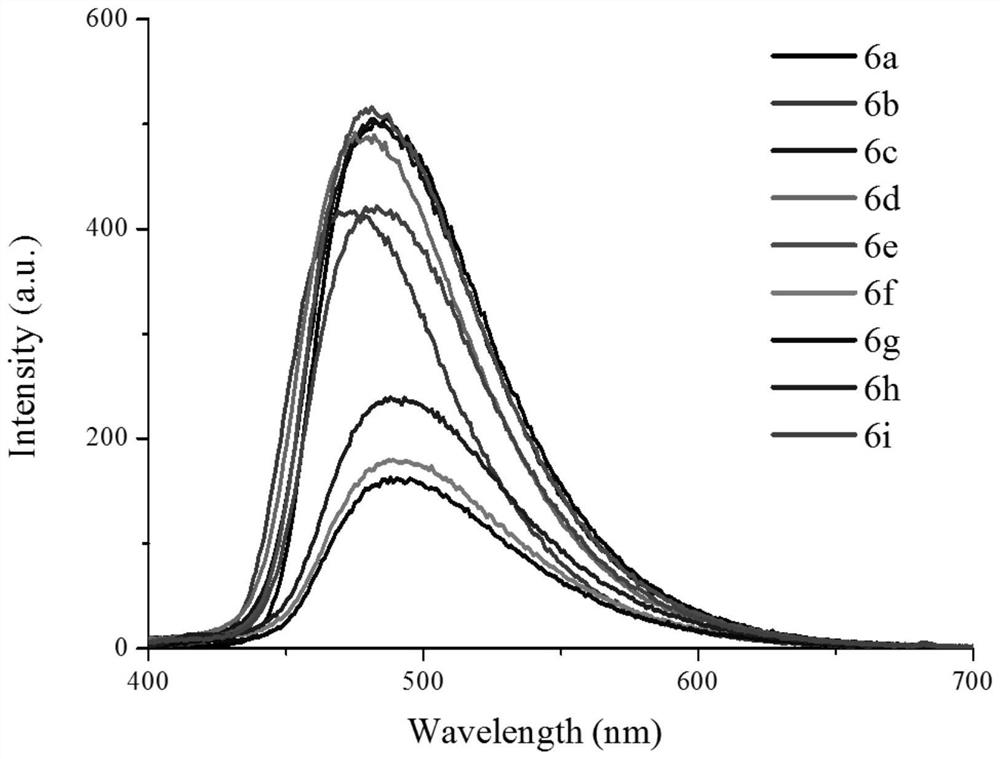
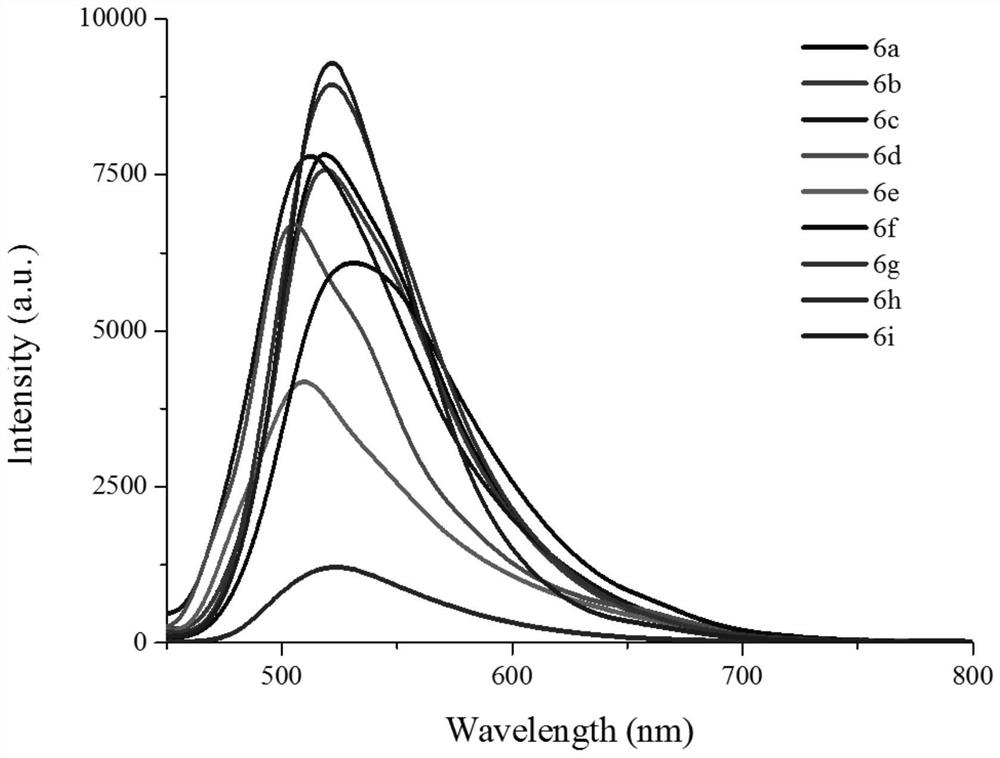
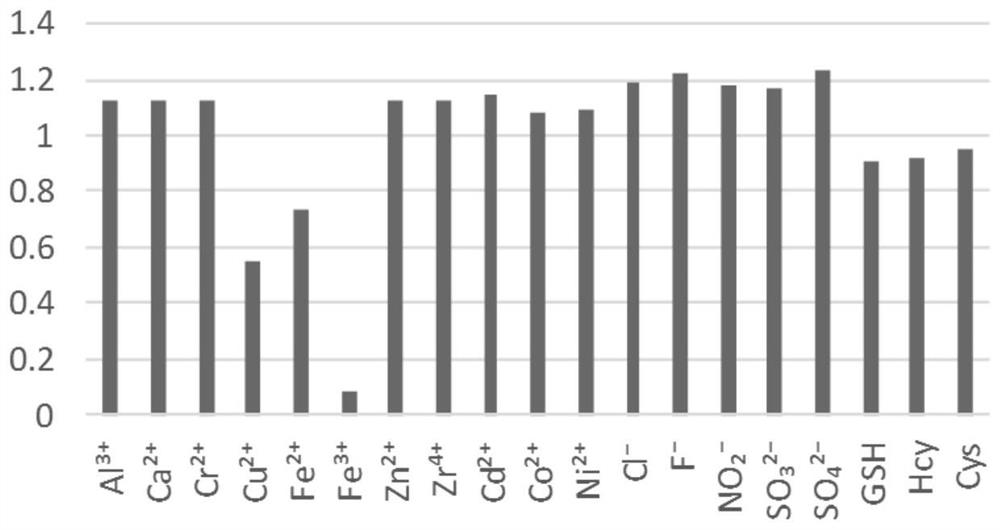
Chromenonaphthyridine-Troger's base Fe<3+> fluorescent probe, and preparation method and application thereof
A naphthyridine and fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve many problems, achieve high yield, mild reaction conditions, and good prospects for large-scale application Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] 1. Chromophthalazine- Synthesis of base derivatives:
[0029]
[0030] (1) Synthesis of Intermediate 2:
[0031] Add 4-bromoaniline (50.0mmol) and paraformaldehyde (100.0mmol) into a 200.0mL round-bottomed flask in sequence, place it in a low-temperature tank and adjust the temperature to -15°C, and slowly add trifluoroacetic acid ( 100.0mL, about 30min dropwise) after reaction at room temperature for 7 days. After the reaction was complete (TLC tracking), the mixture was poured into ice water, adjusted to pH 9-10 with ammonia water, cooled to room temperature, extracted with dichloromethane (50.0 mL×3), and spin-dried to obtain a crude product. Add acetone, heat until the crude product is completely dissolved, recrystallize at room temperature, filter with suction, and wash with acetone to obtain intermediate 2 (12.28 g, 65%).
[0032] (2) Synthesis of Intermediate 3:
[0033] Add Intermediate 2 (5.0mmol) into a 100.0mL round-bottomed flask. After pumping and e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com