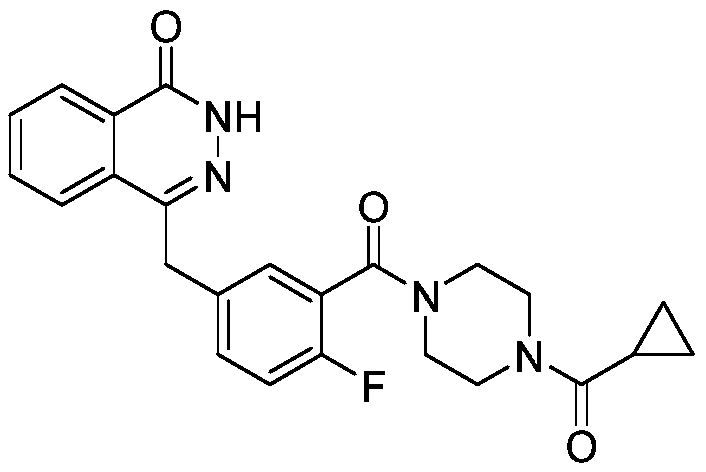
Synthetic method of olaparib
A synthetic method and oxo-generation technology, applied in the direction of organic chemistry, can solve the problems of unfavorable industrial production, difficulty in purification, and high cost of raw materials, and achieve the effects of easy large-scale production, easy control of operating conditions, and mild and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
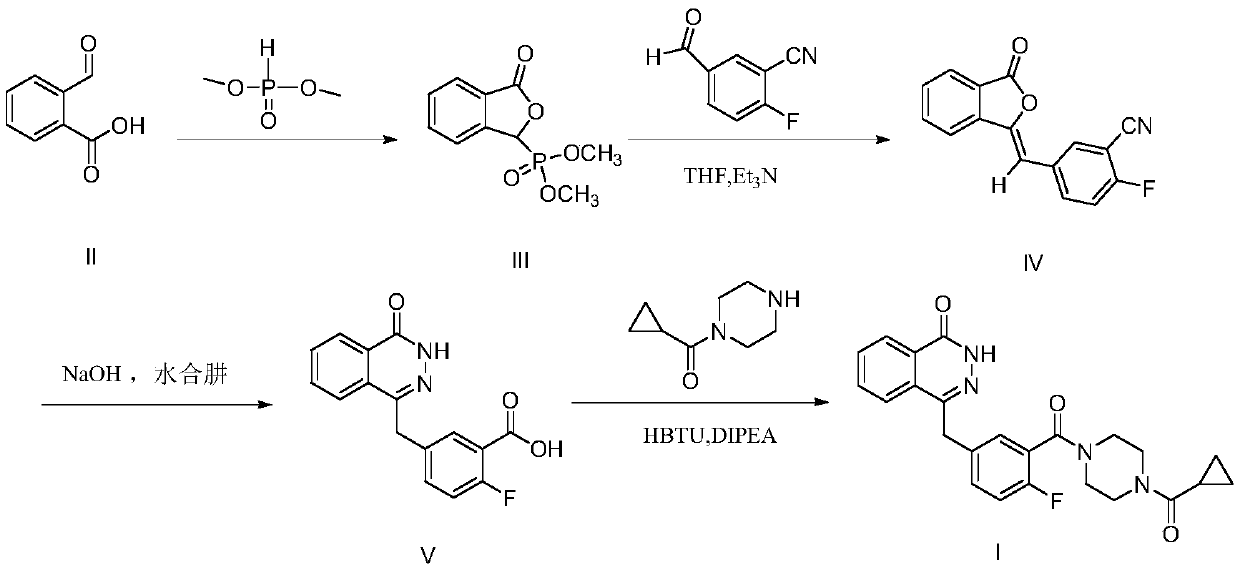
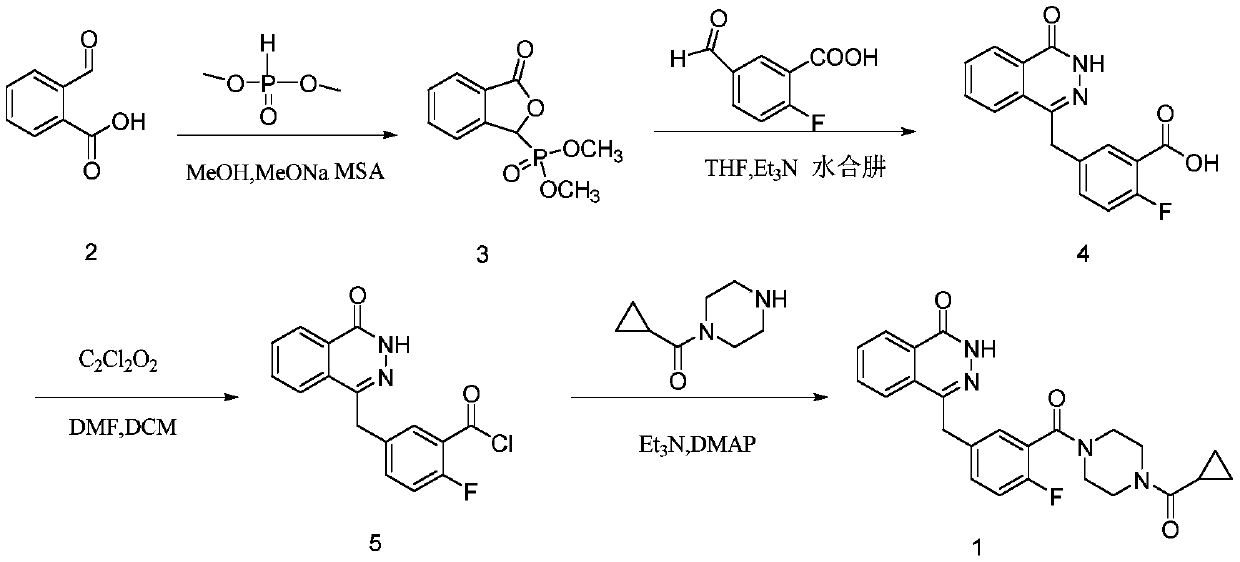
[0052] Preparation of compound 3
[0053] The synthetic route is:
[0054]
[0055] Add sodium formate (1g, 0.018mol) and methanol (16ml) into a round bottom flask, stir, and after cooling down to 0°C, add dimethyl phosphite (2.1ml, 0.022mol) dropwise. O-carboxybenzaldehyde (2.2 g, 0.014 mol) was added slowly, warmed up to room temperature, reacted for 4 h, then methanesulfonic acid (1.3 ml, 0.019 mol) was added dropwise within 10 min, stirred, and concentrated to dryness under reduced pressure. Add water (20ml), extract with dichloromethane, collect the dichloromethane layer, wash with anhydrous Na 2 SO 4 dry. After filtration, it was concentrated under reduced pressure to obtain a white solid (2.6 g, yield 91%).
[0056] The target product compound 3 1 The HNMR data are as follows:
[0057] 1 H-NMR (500MHz, DMSO-d 6 )δ: 3.62(d, J=8Hz, 3H), 3.86(d, J=12Hz, 3H), 6.36(d, J=8Hz, 1H), 7.71(m, 1H), 7.88(m, 2H), 7.98 (m, 1H).
Embodiment 2
[0059] Preparation of compound 4
[0060] The synthetic route is:
[0061]
[0062] Compound 3 (2.6g, 0.011mol) and 2-fluoro-5-formylbenzoic acid (2.1g, 0.012mol) were dissolved in anhydrous tetrahydrofuran (25ml), cooled to 0°C, and triethylamine was slowly added dropwise (1.0ml, 0.007mol), rise to room temperature and react for 5h after dropping, then slowly raise the temperature to 70°C, add hydrazine hydrate (5.1ml, 0.107mol) for 3h, cool down to room temperature, add appropriate amount of hydrochloric acid (2mol / L) adjust the pH to acidity, and no more solids are precipitated. Suction filtration, the product was washed with water and ethyl acetate, and dried to give a yellow solid (1.9g, yield 83%)
[0063] The target product compound 4 1 The HNMR data are as follows:
[0064] 1 H NMR (500MHz, DMSO-d 6 )δ13.13(s,1H),12.60(s,1H),8.29(d,J=7.7Hz,1H),8.00(d,J=8.1Hz,1H),7.92(t,J=7.6Hz, 1H), 7.88–7.81 (m, 2H), 7.60 (ddd, J=7.8, 4.5, 2.4Hz, 1H), 7.26 (dd, J=10.8, 8.4H...
Embodiment 3
[0066] Preparation of Compound 5:
[0067] The synthetic route is:
[0068]
[0069] In a dry environment, compound 4 (1.9g, 0.0063mol) was dissolved in anhydrous dichloromethane under the protection of nitrogen, cooled to 0°C, and oxalyl chloride (2.4ml, 0.025mol) was slowly added dropwise, and rose to At room temperature, add two drops of DMF dropwise, react at room temperature for 2 hours, remove the solvent and excess oxalyl chloride under reduced pressure to obtain 2.1 g of light yellow solid, which is directly put into the next step reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com