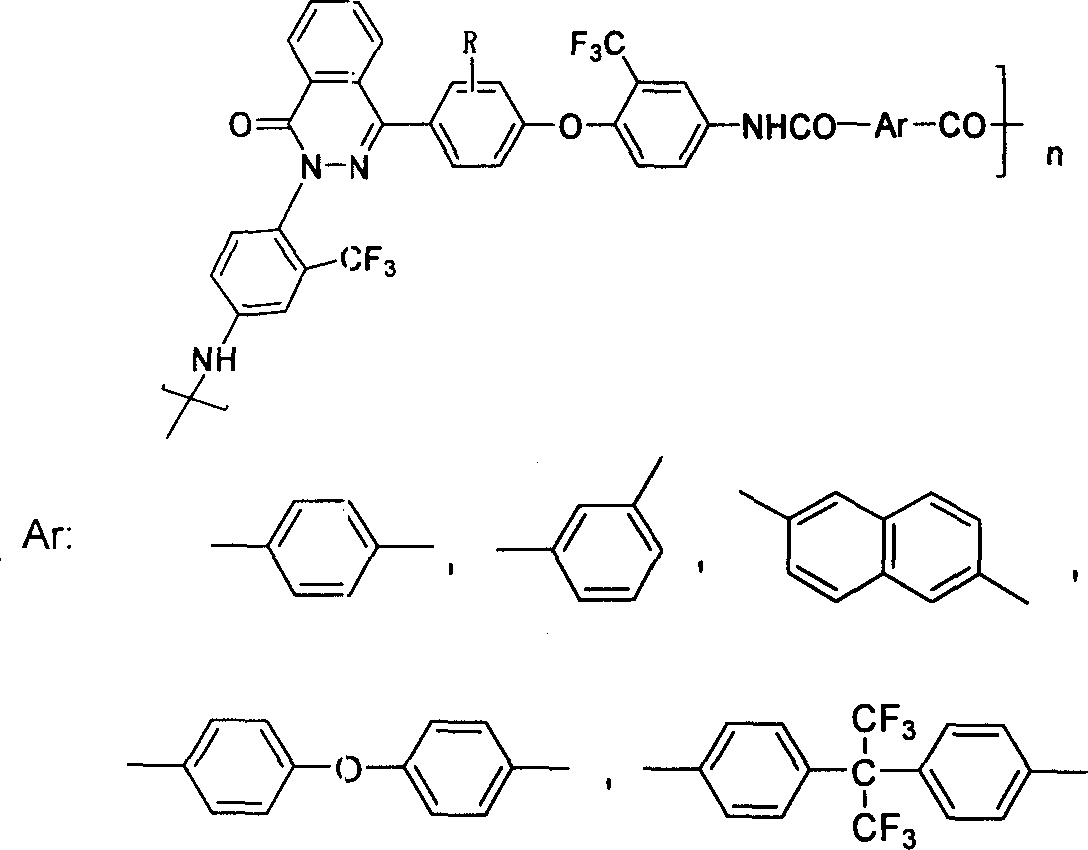
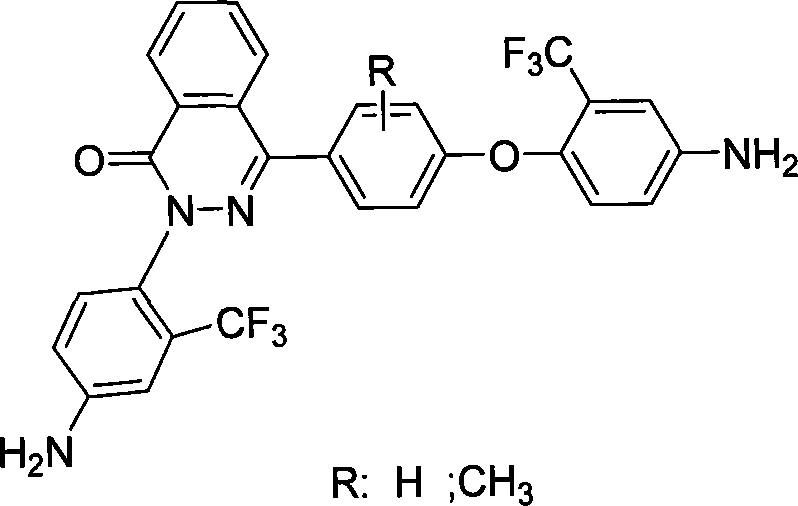
Aromatic polyamide containing fluorine and diamine monomer containing fluorine based on naphthyridine ketone structure and method of producing the same
A technology of fluorine-containing diamine monomer and phthalazinone, which is applied in the field of fluorine-containing polyarylamide compound and its synthesis based on the structure of phthalazinone, can solve the problem of limiting the application of polyarylamide, the inability to melt process, Not suitable for solution processing and other problems, to achieve the effect of improving solubility, increasing rigidity, and good high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] (1) The best example of diamine monomer synthesis
[0025] In a 250ml flask equipped with a mechanical stirrer, condenser, thermometer, and water separator, add 4-(4-hydroxyphenyl)phthalazinone 30mmol, 2-chloro-5-nitrobenzotrifluorotoluene 61.5 mmol, anhydrous potassium carbonate 63mmol, DMAC (N,N-di-methylacetamide) 40ml, heated to 120°C under nitrogen protection, stirred for 12h, poured into 600ml of ice water after cooling, stirred and settled, there were a lot of light yellow solids Precipitate, filter and rinse with warm water several times, and then vacuum-dry the product at 40°C to obtain 16g of dinitro compound with a crude yield of 87%.
[0026] Add dinitro compound 2-(4-nitro-2-trifluoromethyl-phenyl)-4-[2 in the 250ml flask that mechanical stirrer, condenser, thermometer and constant pressure dropping funnel are housed, 3-Dimethyl-4-(4-nitro-2-trifluoromethyl-phenoxy) phenyl] phthalazin-1-one 25mmol, ethylene glycol methyl ether 100ml, catalyst Pd / C ( 0.5%)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com