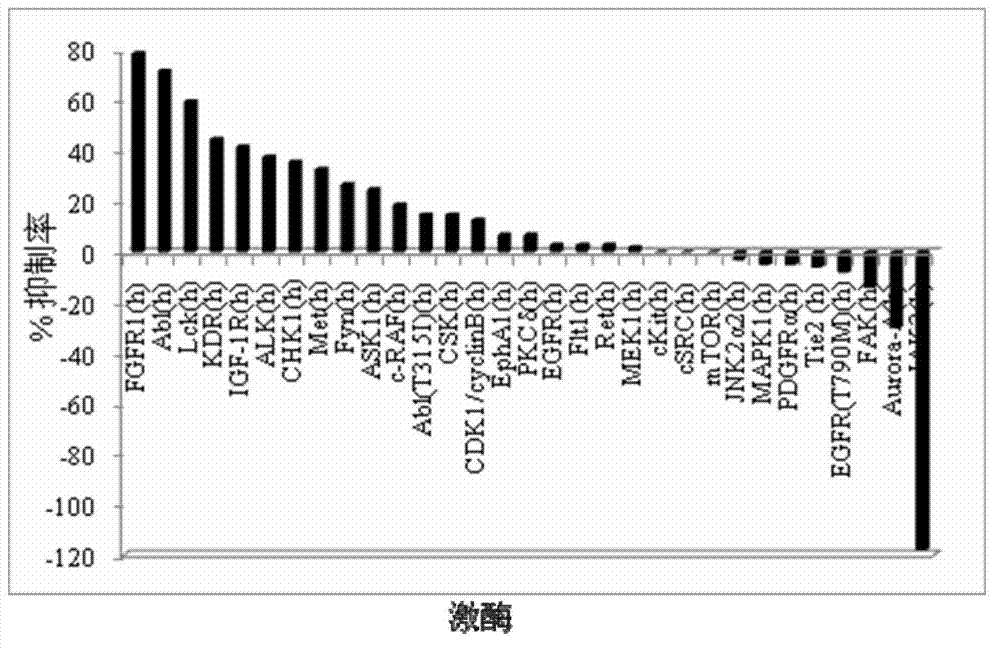
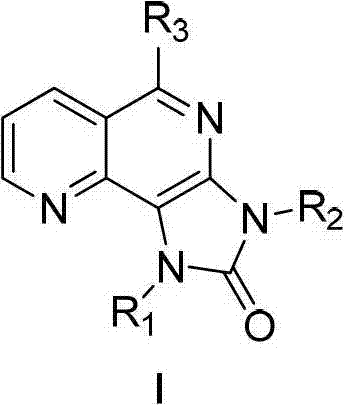
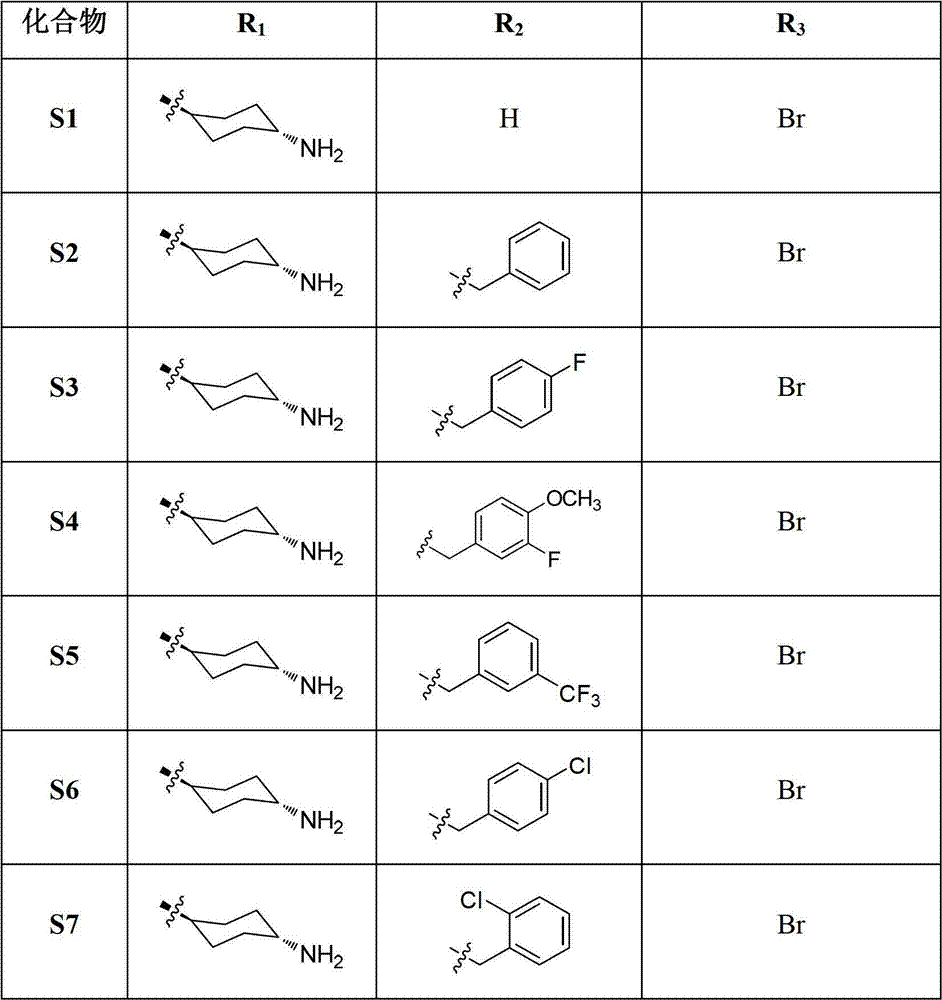
Tri-substituted imidazo diazanaphthalene ketone compounds, preparation method thereof and applications thereof
A compound, imidazo technology, applied in the field of medicinal chemistry, can solve problems such as blocking tumor cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 2
[0064] Preparation Example 2 3-Hydroxymethyl-pyridine-2-carboxylic acid isopropyl ester (4)
[0065]
[0066] Compound 2 (52.7 g, 0.25 mol) was dissolved in 400 mL of thionyl chloride and heated to reflux until the solution became homogeneous, and then the solvent was spin-dried. Add 2 × 50mL anhydrous THF and rotary steam to remove residual thionyl chloride. The obtained red liquid was dissolved in 400 mL of anhydrous THF and cooled to 0° C., sodium borohydride (28.6 g, 0.76 mol) was added in batches, stirred at 0° C. for 4 hours, and the reaction solution was carefully poured into ice water. 3×200mL dichloromethane extraction, add anhydrous Na 2 SO 4 dry. Column chromatography (petroleum ether: ethyl acetate = 3:2) gave compound 4 (18.8 g, 38%) as a yellow solid. 1 H NMR (CDC1 3 ,300MHz):δ8.69(dd,J=1.5,4.7Hz,1H),7.88(dd,J=1.5,7.7Hz,1H),7.46(dd,J=4.7,7.8Hz,1H),5.35( septet,J=6.4Hz,1H),4.81(m,2H),1.45(d,J=6.3Hz,6H);EI-MS m / z:195(M) + .
preparation Embodiment 3
[0067] Preparation Example 3 3-{[Methoxycarbonylmethyl-(toluene-4-sulfonyl)-amino]-methyl}-pyridine-2-carboxylic acid isopropyl ester (5)
[0068]
[0069] Compound 4 (1.734g, 8.89mmol), methyl 2-p-toluenesulfonamidoacetate (TsNHCH 2 COOCH 3 ) (2.163g, 8.89mmol), and triphenylphosphine (3.499g, 13.338mmol) were dissolved in 100mL of anhydrous THF, cooled to 0°C, and protected with nitrogen. DEAD (2.165 mL, 13.338 mmol) was dissolved in 10 mL of anhydrous THF, and DEAD was added dropwise. The ice bath was removed, stirred for two hours and then spin-dried to obtain red oily liquid compound 5, which was directly used in the next reaction.
[0070] Methyl 8-hydroxy-1,6-naphthalene-7-carboxylate (6)
[0071]
[0072] The compound 5 (8.89mmol) obtained in the previous step reaction was dissolved in 50mL of anhydrous methanol and cooled to 0°C. Sodium methoxide (1.681 g, 31.123 mmol) was added slowly. Remove the ice bath and stir for 3 hours. Spin off the solvent, add 20...
preparation Embodiment 4
[0073] Preparation Example 4 5-Bromo-8-hydroxy-1,6-naphthyridine-7-carboxylic acid methyl ester (7)
[0074]
[0075] NBS (30mg, 0.167mmol) was added to 1mLCH of compound 6 (34mg, 0.167mmol) at room temperature 2 Cl 2 solution, stirred for 1 hour. Filtration and drying gave white solid compound 7 (30mg, yield 85%); 1 HNMR(d 6 -DMSO,300MHz):δ9.26(dd,J=1.5,4.2Hz,1H),8.59(dd,J=1.6,8.4Hz,1H),8.00(dd,J=4.2,8.4Hz,1H), 3.94(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com