Polycyclic aromatic hydrocarbon aza-naphthalene derivative, synthesis method and electronic device thereof
A technology for polycyclic aromatic hydrocarbons and electronic devices, which is applied in the field of electronic devices containing polycyclic aromatic hydrocarbons and aziridine derivatives, to achieve high luminous efficiency, improve electron transmission efficiency, and improve luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Embodiment 1: the synthesis of compound 1-10
[0137] (Synthesis of intermediate M1)
[0138] The synthetic route of intermediate M1 is as follows:
[0139]
[0140] P-bromobenzohydrazide (4.3g, 20mmol), acenaphthoquinone (3.6g, 20mmol), ammonium acetate (11.8g, 200mmol) and 150mL acetic acid were successively added into a 250mL single-necked flask, and the reaction was stirred under reflux for 12 hours. After the reaction was complete, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. The crude product was further purified by column chromatography (petroleum ether:dichloromethane=2:1 (V / V)). The solvent was evaporated, and after drying, 4.5 g of an orange solid was obtained, yield 63%. MS(EI): m / z: 360.08[M + ]. Anal.calcd for C 19 h 10 BrN 3 (%): C 63.35, H 2.80, N 11.67; found: C 63.33, H 2.85, N 11.64.
[0141] (Synthesis of Intermediate M2)
[0142] The synthetic route of intermediate M2 is as follows:
...
Embodiment 2
[0149] Embodiment 2: the synthesis of compound 1-34
[0150] (Synthesis of Intermediate M3)
[0151] The synthetic route of intermediate M3 is as follows:
[0152]
[0153] Under an argon atmosphere, 5-bromo-2-chloropyrimidine (1.1 g, 5.7 mmol), M1 (1.8 g, 5.0 mmol) and 50 mL of toluene were successively added to a 100 mL one-necked flask, followed by 0.5 M bis(trimethylsilane A toluene solution of potassium amide (6.0 mmol) was added dropwise to the reaction system. After reacting at 50°C for 12 hours, the reaction was quenched by adding saturated ammonium chloride solution. The organic layer was extracted with ethyl acetate and washed with Na 2 SO 4 After drying, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. The crude product was further purified by column chromatography (petroleum ether:dichloromethane=2:1 (V / V)). The solvent was evaporated, and after drying, 1.4 g of a yellow solid was obtained with a yield of 63...
Embodiment 3
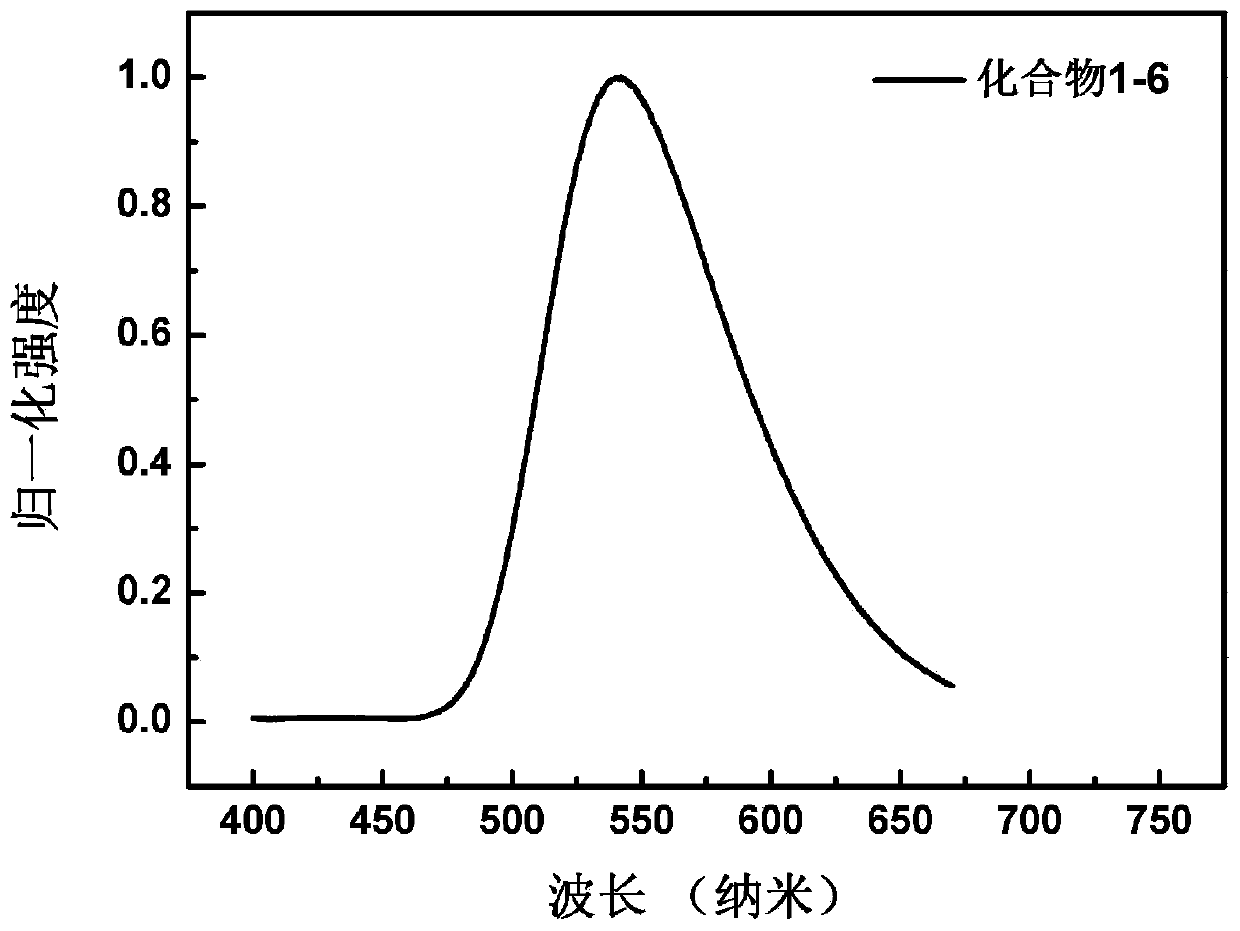
[0158] Embodiment 3: the synthesis of compound 1-6
[0159] (Synthesis of Intermediate M4)
[0160] The synthetic route of intermediate M4 is as follows:
[0161]
[0162] Add benzohydrazide (2.7g, 20mmol), 5,6-dibromoacenaphthenequinone (6.8g, 20mmol), sodium acetate (27g, 200mmol) and 150mL acetic acid successively into a 250mL single-necked flask, and react under reflux for 12 hours. After the reaction was complete, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. After drying, 5.2 g of an orange solid was obtained, yield 59%. MS(EI): m / z: 439.02[M + ]. Anal.calcd for C 19 h 9 Br 2 N 3 (%): C 51.97, H 2.07, N 9.57; found: C 51.95, H 2.10, N 9.54.
[0163] (Synthesis of Intermediate M5)
[0164] The synthetic route of intermediate M5 is as follows:
[0165]
[0166] Under an argon atmosphere, 5-bromopyrimidine (0.9 g, 5.7 mmol), M4 (2.2 g, 5.0 mmol) and 50 mL of toluene were successively added to a 100 mL sing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com