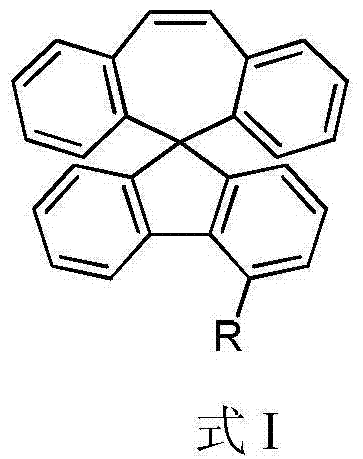
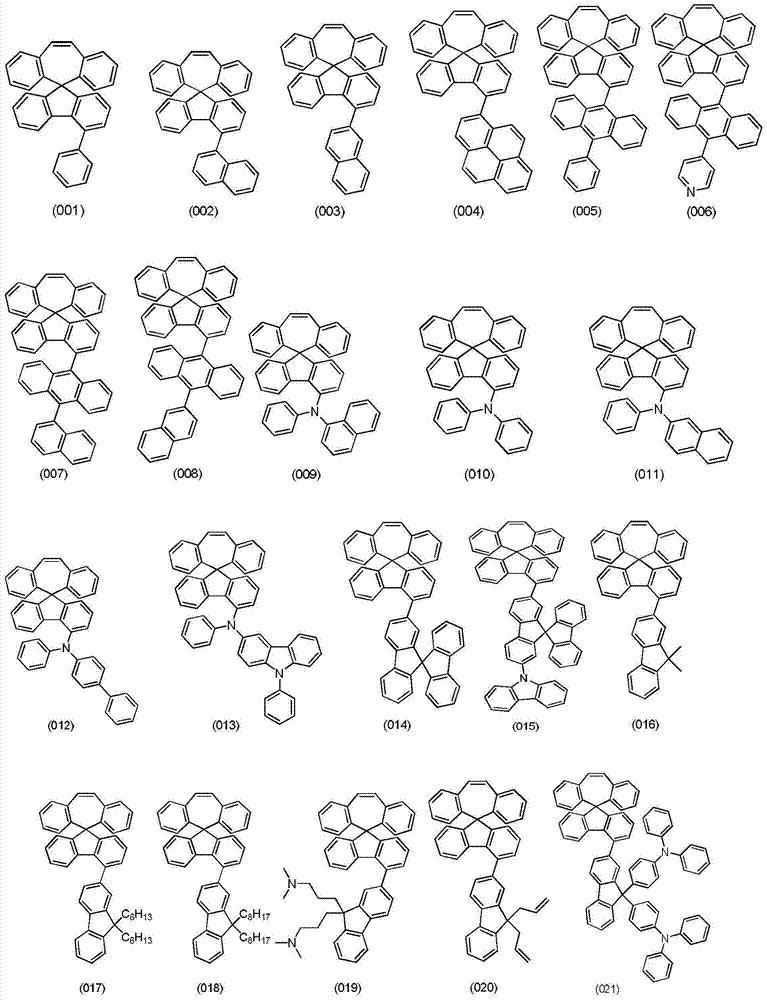
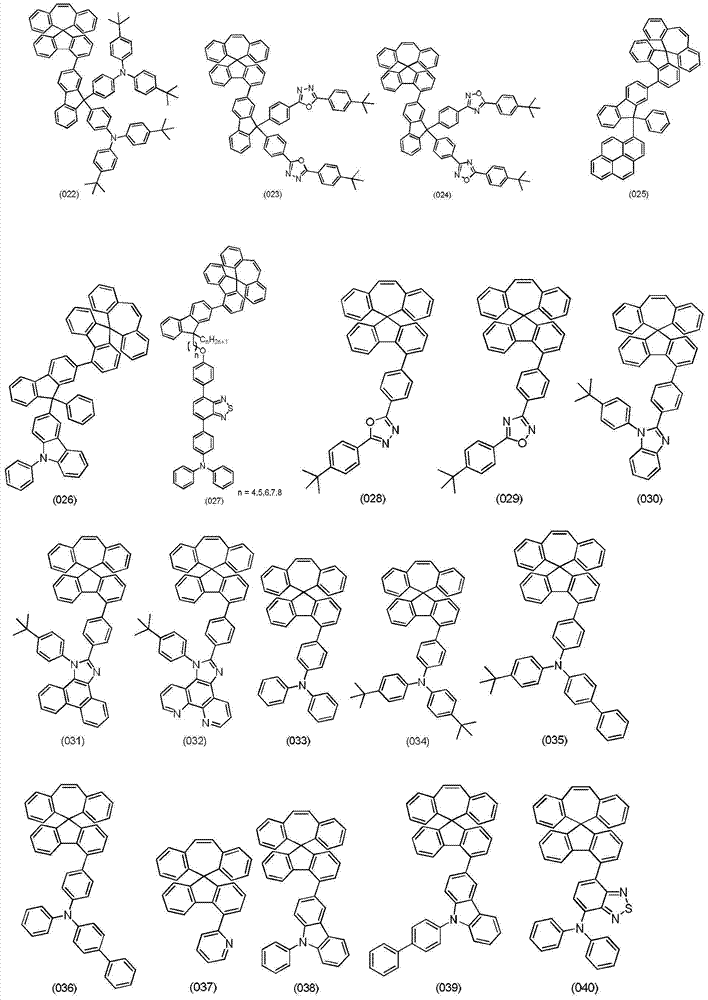Organic electroluminescent materials, and preparation method and application thereof
A technology of luminescent materials and compounds, applied in the direction of luminescent materials, organic chemistry, silicon organic compounds, etc., to achieve the effects of lower starting voltage, good film-forming performance, good fluorescence quantum efficiency and electroluminescent efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1, the preparation of compound 006
[0075]
[0076] The first step: preparation of compound S-3
[0077]
[0078] 4.2g of S-2 was dissolved in 150ml of dry DMF, 2.8g of diboronic acid pinacol ester, 1.2g of anhydrous potassium acetate and 15mg of Pd(dppf)Cl were added 2 Catalyst, under the protection of nitrogen, heat up to 100 ° C, stir for 2 hours, cool to room temperature, pour the reaction solution into 500 ml of ice water, filter with suction, wash the filter cake with water, separate and purify the obtained solid with a silica gel column, and obtain 4.4 g of S-3, white solid.
[0079] The second step: the preparation of 4-(10-bromoanthracen-9-yl)pyridine
[0080]
[0081] 7.7 g of 4-pyridineboronic acid was dispersed with 50 ml of methanol and 150 ml of water, and 12.2 g of potassium bifluoride was added, and stirred and reacted at room temperature for 2 hours to obtain a clear solution, which was concentrated to dryness under reduced pressu...
Embodiment 2
[0090] Embodiment 2, the preparation of compound 009
[0091]
[0092] Take 2g of compound S-2 and 1.25g of N-phenyl-1-naphthylamine, 684mg of anhydrous sodium tert-butoxide, and 80ml of toluene, then add 50mg of tris(dibenzylideneacetone) dipalladium catalyst and 40mg 2-dicyclohexylphosphine-2′,6′-dimethoxybiphenyl, reflux under nitrogen protection overnight, cool to room temperature, add 50ml of water, separate the organic phase, extract the aqueous phase with DCM, organic phase reuse MgSO 4 After drying and suction filtration, the filtrate was concentrated to dryness under reduced pressure, and the residue was separated and purified by silica gel column to obtain 2.1 g of compound 009 as a white solid.
[0093] Experimental data:
[0094] (1) 1 HNMR (δ, CDCl 3 ): 6.62~6.65 (1H, q); 6.79~7.21 (17H, m); 7.34~7.45 (5H, m); 7.69~7.73 (3H, m); It is confirmed that the substance obtained by the reaction is indeed compound 009
[0095] (2) Glass transition temperature Tg...
Embodiment 3
[0098] Embodiment 3, the preparation of compound 033
[0099]
[0100] The first step: the preparation of 4-bromotriphenylamine
[0101]
[0102] Disperse 27.2g of triphenylamine in 500ml of carbon tetrachloride, add 21.4g of NBS, raise the temperature and reflux and stir for 4 hours, cool to room temperature, filter with suction, concentrate the filtrate to dryness under reduced pressure, and recrystallize the residue with ethanol to obtain 30g 4-Bromotriphenylamine, white solid.
[0103] The second step: the preparation of N,N-diphenyl-4-(boronic acid pinacol ester) aniline
[0104]
[0105] With reference to the method in the first step of Example 1, S-2 is replaced with 4-bromotriphenylamine and 1.1 molar equivalents of pinacol ester of diboronic acid, 1.5 molar equivalents of anhydrous potassium acetate, 1% mole (with 4-bromotriphenylamine Triphenylamine as a base) palladium catalyst, and finally N,N-diphenyl-4-(boronic acid pinacol ester group) aniline, yellow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com