Near-infrared boron dipyrromethene (BODIPY) compound based on duplex heterocyclic pyrrole group, and preparation method and application of BODIPY compound
A technology of fluoroborate dipyrrole and pyrrole group, which is applied in the field of preparing photosensitizers in photodynamic therapy, can solve the problems of low absorption intensity, no test cell photodynamic therapy effect, low efficiency of BODIPY, etc., and achieve triplet efficiency increase Large, attractive application prospects, and the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]The synthesis of embodiment 1.BODIPY-A1:
[0041]
[0042] Add 1mmol (164mg) p-methoxyformylbenzaldehyde, 2mmol (410mg) dithienopyrrole, and 40ml anhydrous dichloromethane into a 100ml round bottom flask, put in a magnet and start stirring, avoid light and protect with argon Inject a drop of trifluoroacetic acid (TFA) into the lower syringe, and react at room temperature for 12 hours. After that, add 1mmol DDQ (227mg), react for 1 hour, then add 2ml triethylamine (TEA) and 2ml BF every ten minutes 3 ·Et 2 O, a total of 3 times, then stirred for 1 hour. Water quenching reaction, NaHCO 3 Wash with water, saturated brine successively, anhydrous Na 2 SO 4 Dry, evaporate the solvent under reduced pressure to obtain a black powder, use 100-140 mesh silica gel column, ethyl acetate-petroleum ether as eluent for chromatographic separation, evaporate the solvent to a golden solid, the yield is 95%, after chloroform and Golden crystals were obtained after recrystallizatio...
Embodiment 2
[0045] The synthesis of embodiment 2.BODIPY-A2, A3, A4:
[0046]
[0047]
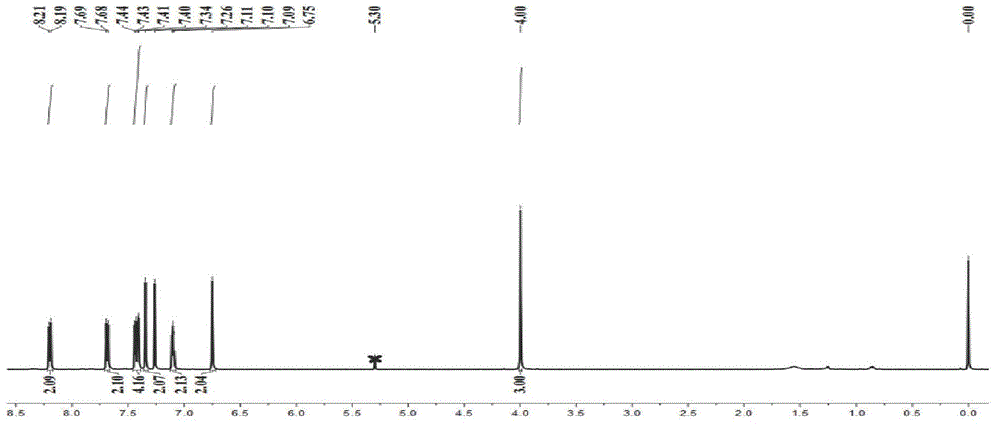
[0048] 0.17mmol (100mg) of BODIPY-A1 prepared in Example 1, 1.02mmol (182mg) of NBS and 30ml of tetrahydrofuran (THF) were added to a 100ml bottom flask, and stirred at room temperature for 6 hours. Sodium thiosulfate solution quenched the reaction, washed with water and saturated brine successively, anhydrous Na 2 SO 4 Dry, evaporate the solvent under reduced pressure to obtain a black powder, use a 100-140 mesh silica gel plate, 20% ethyl acetate / petroleum ether for chromatographic separation, and obtain BODIPY-A2, A3, and A4 with yields of 23% and 57% respectively , 20%. BODIPY-A2 UV 693nm, emission 728nm. MALDI-TOF-MS m / z:calcd760.842,found:758.402[M-2H] + ,737.428[M-H 3 F] + . BODIPY-A3 UV 698nm, emission 724nm. MALDI-TOF-MS m / z:calcd839.213,found:838.219[M-H] + ,821.072[M-F] + . BODIPY-A4 UV 692nm, emission 727nm. MALDI-TOF-MS m / z:calcd 918.110,found:917.235[M-H] + ,898.676[M-H...
Embodiment 3
[0051] The synthesis of embodiment 3.BODIPY-A5:
[0052]
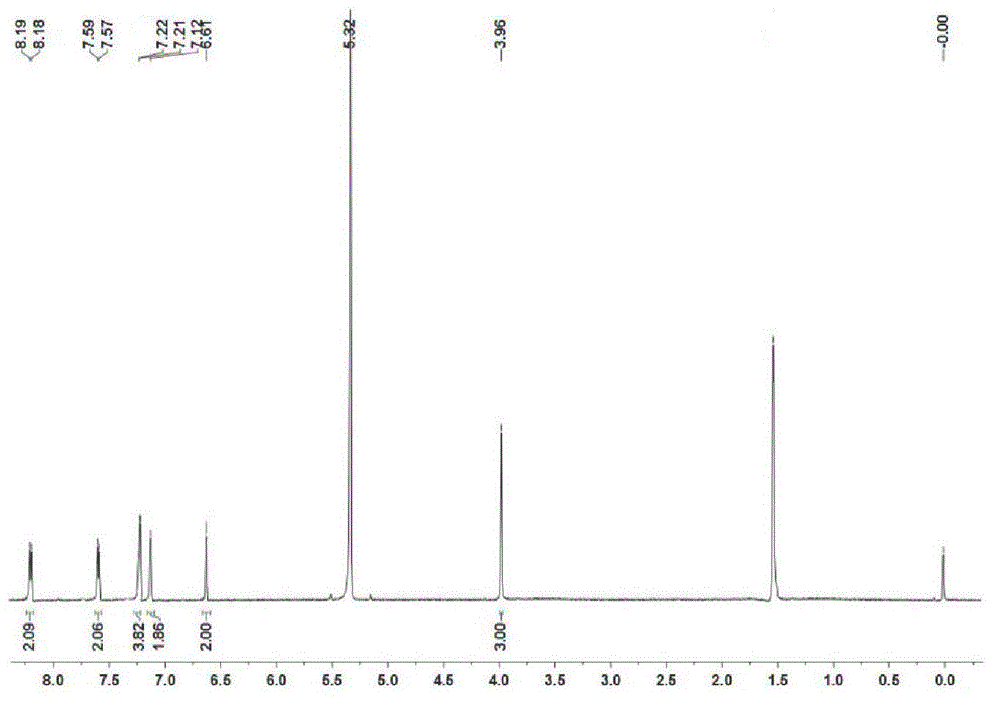
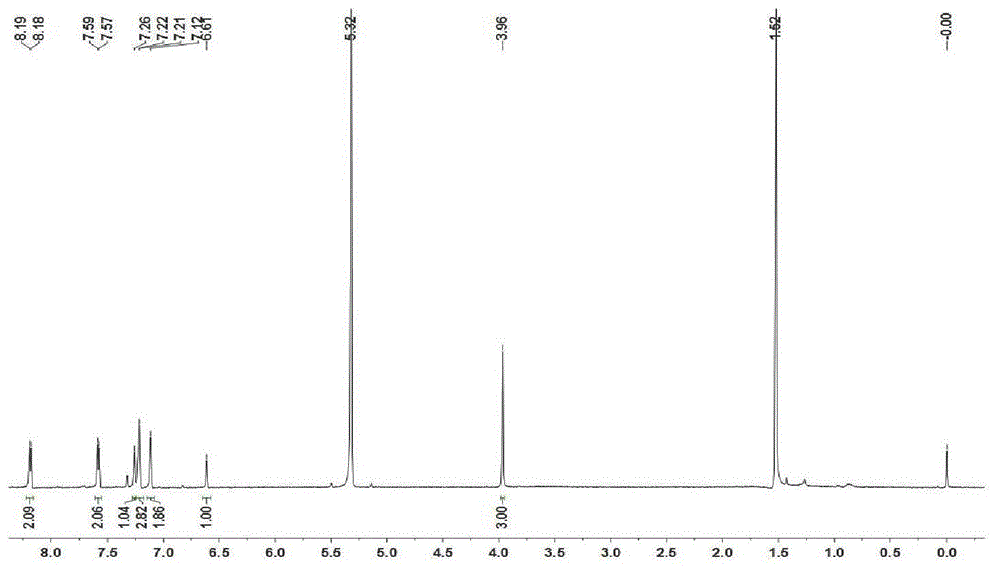
[0053] In a 100 ml bottom flask add 0.17 mmol (100 mg) BODIPY-A1, 17.0 mmol (2.72 g) NBS and 30 ml CHCl 3 , stirred at room temperature for 1 hour. Quenched reaction with NaOH solution, washed with water and saturated brine successively, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a black powder, which was packed into a 100-140 mesh silica gel column with chloroform as the eluent for chromatographic separation, and evaporated to dryness to obtain a reddish-brown powder. Yield 93%. UV: 633nm, Emission: 723nm. MALDI-TOF-MS m / z: calcd1233.694, found: 1212.405 [M-H 2 F] + ,1152.502[M-H 2 Br] + . NMR spectrum see attached image 3 . See Figure 5(d) for the mass spectrum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com