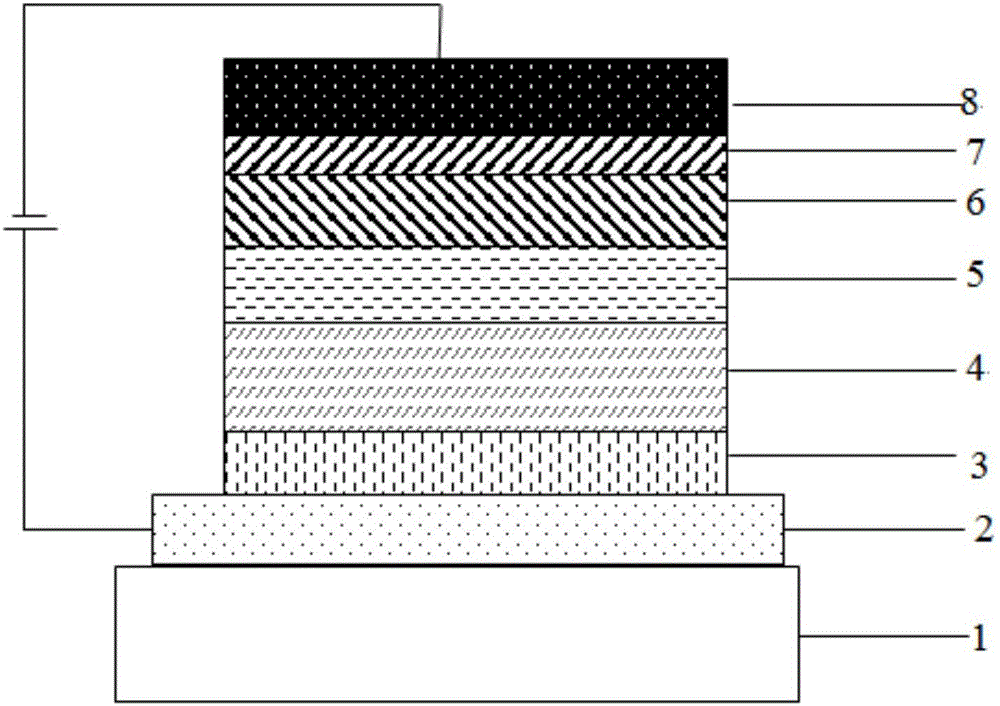
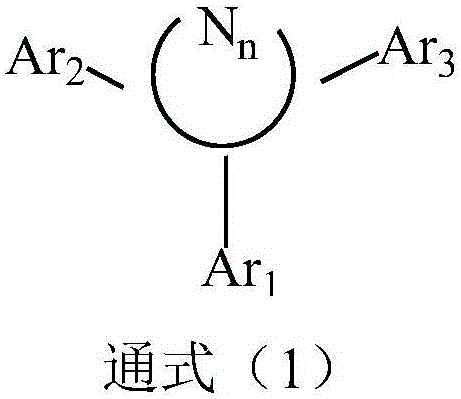
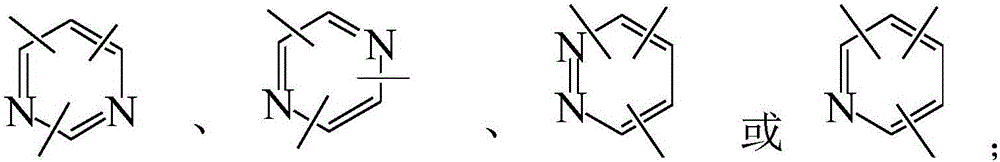Compound with pyridine as core and application thereof in organic electroluminescence device
A compound, azabenzene technology, applied in the application field of organic electroluminescent devices, can solve the problems of low S1 state radiation transition rate, efficiency roll-off, difficult exciton utilization rate and high fluorescence radiation efficiency, etc. Film formation and fluorescence quantum efficiency, increasing orbital overlap, avoiding the effect of aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Compound 1
[0046]
[0047] In a 250ml four-neck flask, under a nitrogen atmosphere, add 0.01mol 4,6-dibromo-2-phenyl-pyrimidine, 0.025mol 9,10-dihydro-9,9-dimethylacridine, 0.03mol sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampled on a plate, and the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 99.2% and a yield of 66.00%.
[0048] Elemental analysis structure (molecular formula C 40 h 34 N 4 ): theoretical value C, 84.18; H, 6.00; N, 9.82; test value: C, 84.19; H, 6.04; N, 9.77.
[0049] HPLC-MS: The molecular weight of the material is 570.28, and the measured molecular weight is 570.80.
Embodiment 2
[0050] Example 2 Compound 2
[0051]
[0052] Add 0.01mol 3-biphenyl-4-yl-2,5-dibromo-pyrazine, 0.025mol 10H-phenoxazine, 0.03mol sodium tert-butoxide to a 250ml four-necked flask under nitrogen atmosphere , 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampled and plated, the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 96.8% and a yield of 68.30%.
[0053] Elemental analysis structure (molecular formula C 40 h 26 N 4 o 2 : Theoretical value C, 80.79; H, 4.41; N, 9.42; O, 5.38; Test value: C, 80.82; H, 4.40; N, 9.41;
[0054] HPLC-MS: The molecular weight of the material is 594.21, and the measured molecular weight is 594.62.
Embodiment 3
[0055] Example 3 Compound 3
[0056]
[0057] Add 0.01mol 4-biphenyl-4-yl-3,6-dibromo-pyrazine, 0.025mol 10H-phenoxazine, 0.03mol sodium tert-butoxide to a 250ml four-necked flask under nitrogen atmosphere , 1×10 -4 mol Pd 2 (dba) 3 , 1×10 -4 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling plate, the reaction was complete; naturally cooled, filtered, the filtrate was rotary evaporated, and passed through a silica gel column to obtain the target product with a purity of 95.0% and a yield of 72.30%.
[0058] Elemental analysis structure (molecular formula C 40 h 26 N 4 o 2 : Theoretical value C, 80.79; H, 4.41; N, 9.42; O, 5.38; Test value: C, 80.80; H, 4.36; N, 9.47;
[0059] HPLC-MS: The molecular weight of the material is 594.21, and the measured molecular weight is 594.69.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com