Preparation method of diaza-naphthalenone-biphenyl-polybenzoxazole, monomer and polymer
A technology of phthalazinone biphenyl polybenzoxazole and xinaphthinone biphenyl polybenzoxazole, which is applied in the field of high-performance polymer materials, can solve the problems of time and energy consumption, complex synthesis process, trichloro Benzene toxicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
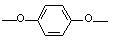
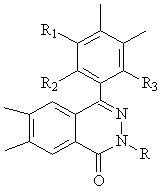
[0082] In a reactor equipped with mechanical stirring, under the protection of nitrogen, add 3.5kg of 80% polyphosphoric acid, 2-(3-hydroxyl-4-aminophenyl)-4-[4-(3-amino-4- hydroxyphenoxy)]-2,3-naphthyridine-1-one (a, R 1 =R 2 =R 3 =R 4 =H) 0.500mol, terephthalic acid 0.500mol, add phosphorus pentoxide 0.12 kg, start stirring. The temperature was raised to carry out the polymerization reaction, and the reaction was carried out at 80°C for 2 hours, at 120°C for 8 hours, at 150°C for 10 hours, and at 185°C for 10 hours to obtain a viscous liquid. After the reaction is over, cool down to about 80°C, add deionized water to wash fully, filter, wash repeatedly with deionized water and dilute sodium bicarbonate solution until neutral, and vacuum dry at 100°C.
[0083] The chemical structure of the polymer was confirmed by infrared spectroscopy, the molecular weight was confirmed by high-performance gel chromatography (GPC) analysis, and the thermal stability was confirmed by ther...
Embodiment 2
[0085] The method is basically the same as in Example 1, wherein the raw material adopts four-functionality monomer b, c or d to replace 2-(3-hydroxyl-4-aminophenyl)-4-[4-(3-amino-4- hydroxyphenoxy)]-2,3-naphthyridine-1-one (a, R 1 =R 2 =R 3 =R 4 = H); other aromatic diacids or diacid chlorides other than terephthalic acid instead of terephthalic acid; 2-(3-hydroxy-4-aminophenyl)-4-(3-amino-4-hydroxyphenyl )-naphthyridine-1-one (a) and terephthalic acid in a molar ratio of 0.98-1.3:1 instead of 1:1; the solvent uses p-toluenesulfonic acid or concentrated sulfuric acid instead of polyphosphoric acid to make the solution mass concentration is 5-30%; the amount of phosphorus pentoxide shrinking agent is to make the solvent mass concentration 60-120%; the sedimentation agent is methanol, ethanol, alkali aqueous solution or a mixture of the above two, and the alkali aqueous solution is NaOH, KOH, Na 2 CO 3 , NaHCO 3 or K 2 CO 3 aqueous solution to replace deionized water; ...
Embodiment 3
[0088] In a reactor equipped with mechanical stirring, under the protection of nitrogen, add 1.5kg of 85% polyphosphoric acid, 2-methyl-4-(3-amino-4-hydroxyphenyl)-6-amino-7-hydroxy -2,3-naphthyridine-1-one (b, R 1 =R 2 =R 3 =R 4 =H) 0.250 mol, terephthalic acid 0.500 mol, 4,6-diaminoresorcinol hydrochloride 0.250 mol, 0.12 kg phosphorus pentoxide, start stirring, and heat up for polymerization. React at 100°C for 5 hours, 120°C for 10 hours, 150°C for 10 hours, and 185°C for 6 hours to obtain a viscous liquid. After the reaction is over, cool down to about 80°C, add deionized water to wash fully, filter, wash repeatedly with deionized water and dilute sodium bicarbonate solution until neutral, and vacuum dry at 100°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com