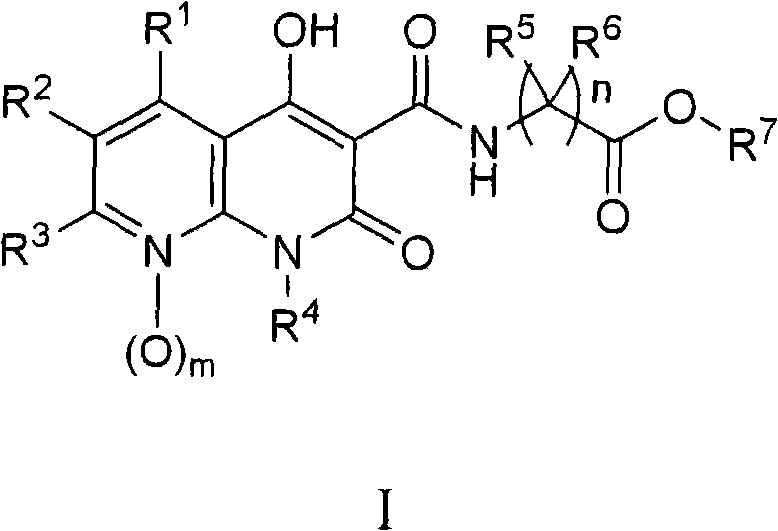
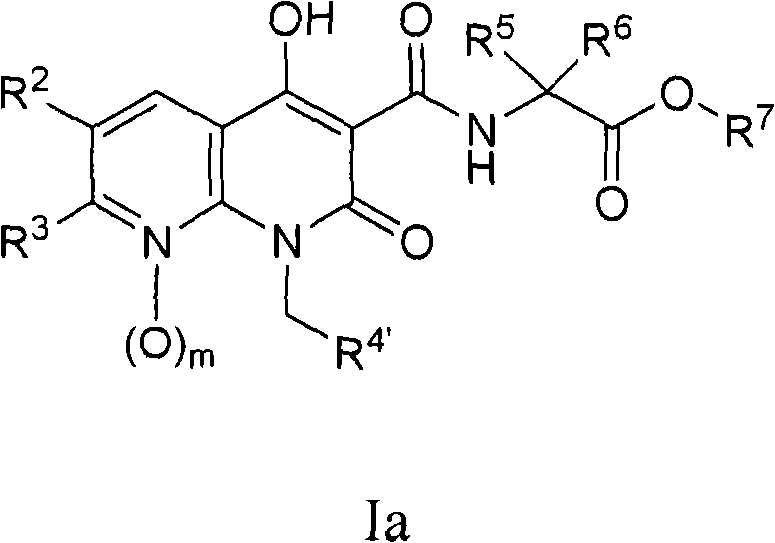
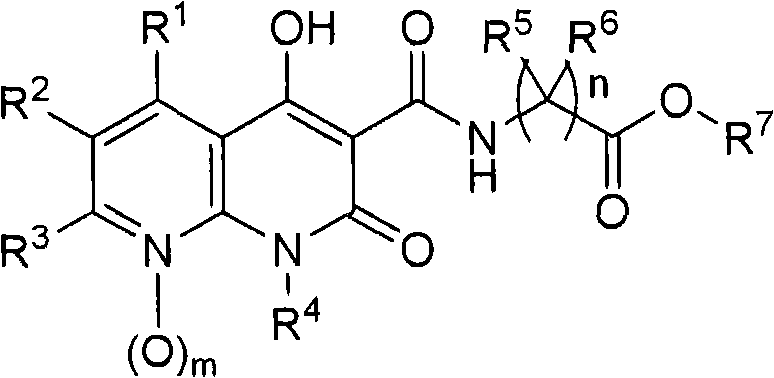
Novel 1,8-naphthyridine compounds
A compound and solvate technology, applied in the field of new 1,8-naphthyridine compounds, can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0166] The preparation of the compounds of the present invention is illustrated in the following schemes. Other synthetic methods are well known to those skilled in the art. The examples describing the preparation of compounds represented by formula I and similar compounds are not to be considered as limiting the invention set forth in the appended claim description. Unless otherwise stated, all variants are as previously defined.
[0167]Intermediates useful in the preparation of compounds of the invention are materials known in the art or can be prepared using chemistry known to those skilled in the art. Examples of reported intermediates and methods for their preparation include ethyl 1-benzyl-4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxylate (IIa) and related substituted analogs (IIb) (see WO 2005 / 021546); 1-alkyl-4-hydroxy-7-methyl-2-oxo-1,2-dihydro-1,8-naphthyridine - 3-ethyl carboxylate analog (IIc) (see Kuroda et al., "Journal of Medicinal Chemistry", 1992...
Embodiment 1
[0185] N-({4-Hydroxy-2-oxo-1-[4-(trifluoromethyl)benzyl]-1,2-dihydro-1,8-naphthyridine-3-yl}carbonyl) Glycine
[0186]
[0187] Step A: 2H-Pyrido[2,3-d][1,3]oxazine-2,4(1H)-dione
[0188]
[0189] 2-(Carbamoyl)nicotinic acid (3.2 g, 19.26 mmol) was dissolved in DMF (30 mL), and lead tetraacetate (8.5 g, 19.26 mmol) was added to the solution in small portions at 0°C. The resulting solution was stirred and allowed to come to room temperature. The reaction mixture was heated at 55°C for one hour, then quenched with water (30 mL). The precipitate formed was filtered off, washed with water and dried to give 2.72 g of the title compound as a solid. 1 H NMR (500MHz, DMSO-d 6 ) δ 12.27 (s), 8.65 (d, 1H, J=4.3Hz), 8.29 (d, 1H, J=7.5Hz), 7.31 (d, 1H, J=7.3Hz).
[0190] Step B: 1-[4-(Trifluoromethyl)benzyl]-2H-pyrido[2,3-d][1,3]oxazine-2,4(1H)-dione
[0191]
[0192] 5.0 g (30.5 mmol) of the compound obtained in step A was dissolved in 50 mL of dimethylacetamide, and 1.46 ...
Embodiment 2
[0201] N-{[1-(4-Chlorobenzyl)-4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthalene-3-yl]carbonyl}glycine
[0202]
[0203] The title compound was prepared in a similar manner to Example 1, substituting 4-(chloro)benzyl bromide for 4-(trifluoromethyl)benzyl bromide in step B: MS: m / z 388 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com