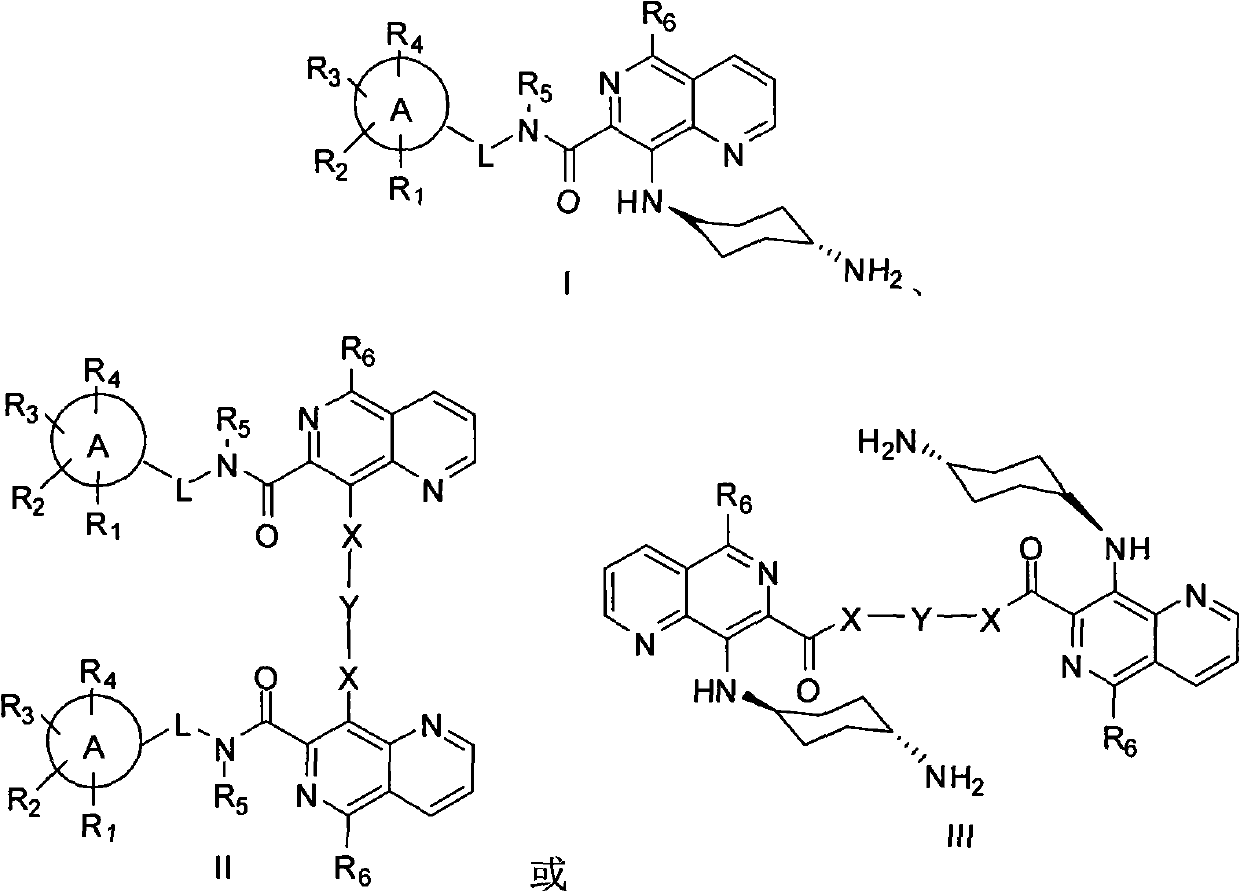
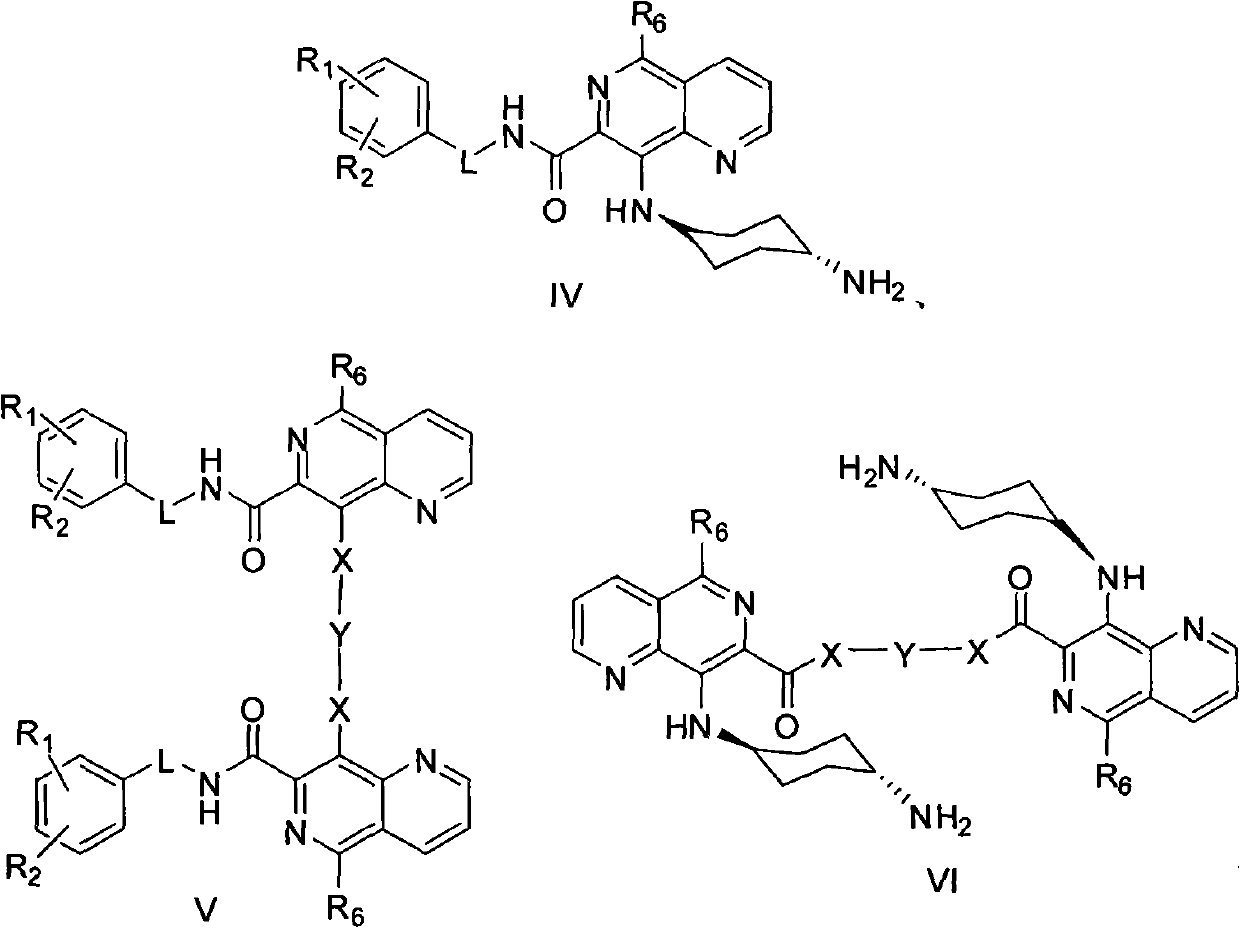
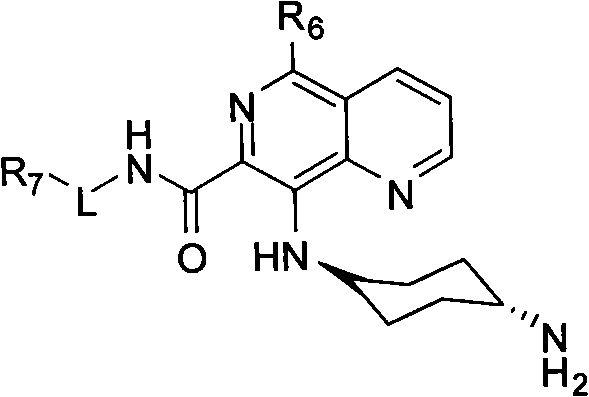
5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, dimer compounds of 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds, and preparation method and use of 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds and dimer compounds of 5,8-disubstituted-1,6-naphthyridine-7-carbonyl amide compounds
A technology of naphthalene and compound, which is applied to 5,8-disubstituted-1,6-naphthalene-7-carbonamide compound and dimer compound thereof. Block tumor cell growth and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] The preparation method of compound S2-1 is similar to that of compound 8-1, except that p-3-chlorobenzylamine is used instead of 2-chlorobenzylamine. Yellow solid, yield: 75-85%. 1 H NMR (300MHz, CDCl 3 ): δ9.20(d, J=4.2Hz, 1H), 8.55(d, J=8.4Hz, 1H), 8.20(m, 1H), 7.73(dd, J=4.2, 8.4Hz, 1H), 7.38 (s, 1H), 7.30 (m, 1H), 7.22 (m, 3H), 4.68 (d, J=6.3Hz, 2H).
[0103] Toluene-4-sulfonic acid 5-bromo-7-(3-chloro-benzylcarbamoyl)-1,6-naphthalene-8-carboxylate (S2-2)
[0104]
[0105] The preparation method of compound S2-2 is similar to that of compound 9-1, except that compound S2-1 is used instead of compound 8-1. White solid, yield: 85-95%. 1 H NMR (CDCl 3 ): δ9.04(d, 1H, J=4.2Hz), 8.57(d, 1H, J=8.4Hz), 8.01(m, 1H), 7.92(d, 2H, J=8.4Hz), 7.69(dd , 1H, J=4.2, 8.4Hz), 7.36-7.28(m, 6H), 4.61(d, 2H, J=6.6Hz), 2.46(s, 3H).
[0106] 8-((1r,4r)-4-aminocyclohexylamino)-N-(3-chloro-benzyl)-5-bromo-1,6-naphthyridine-7-carboxylic acid amine (S2)
[0107]
[0108] The pre...
experiment Embodiment 1
[0437] Experimental example 1: Inhibitory effect of 5,8-disubstituted-1,6-naphthyridine-7-carbonamide compounds and their dimer compounds on the proliferation of human cancer cell lines in vitro
[0438] Cell lines: human breast cancer cell line MDA-MB-435, human ovarian cancer cell line SK-BR-3, human malignant melanoma cell line A375, human skin squamous cell line A431, human colon cancer cell line HT-29, Human lung cancer cell line A-549, human liver cancer cell line BEL-7402, human pancreatic cancer cell line BXPC3, human acute myeloid leukemia cell line HL-60, and human prostate cancer cell line PC-3 were purchased from American Standard Biological Collection .
[0439] Method: sulforhodamine B (SRB) method, specifically as follows: a certain number of different types of tumor cells in the logarithmic growth phase were inoculated in 96-well culture plates, and after 24 hours of cell attachment, different concentrations of For the test compound of the present invention, t...
Embodiment 2
[0466] Example 2: Inhibition of 5,8-disubstituted-1,6-naphthyridine-7-carbonylamides and their dimer compounds on five protein tyrosine kinases
[0467] Protein tyrosine kinase: c-Src and EGFR are expressed by the insect baculovirus expression system in our laboratory, and the active intracellular kinase domain tyrosine kinase protein obtained by affinity purification with Ni-NTA column has been detected Meet the experimental requirements, subpackage at -70°C, and store. KDR, Flt-1 were purchased from Upstate Company (Waltham, MA, USA); ErbB2 was purchased from Calbiochem Company (Darmstadt, Germany).
[0468] Experimental method: The enzyme-linked immunosorbent assay (ELISA) method was used for the test of tyrosine kinase activity, as follows: first, the enzyme reaction substrate Poly(Glu, Tyr) 4:1 (20 μg / mL, overnight at 37°C) was coated with the enzyme Standard plates, washed and dried for later use. When performing the reaction, add 80 μL of ATP solution diluted with rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com