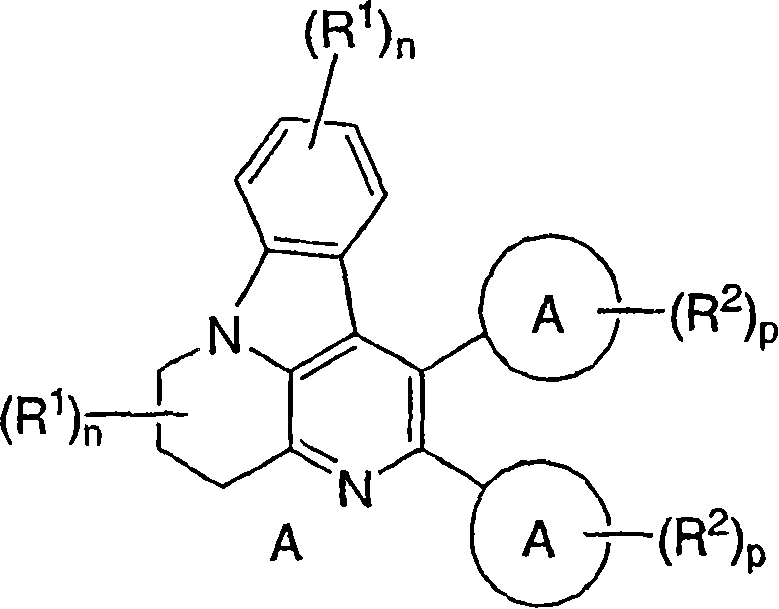
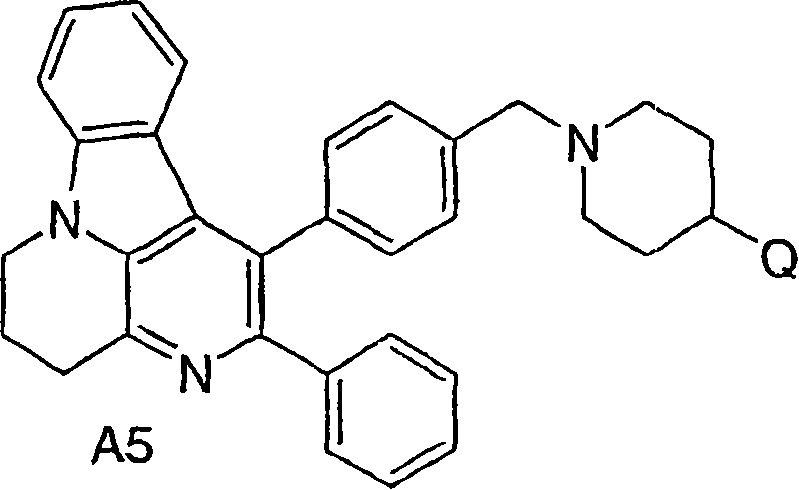
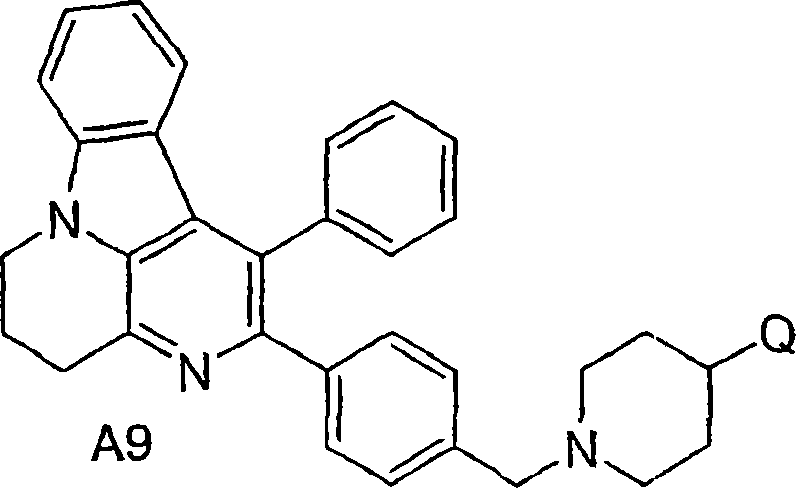
Inhibitors of AKT activity
A technology selected from compounds, applied to one or more activity inhibitors of the serine/threonine kinase Akt isoenzyme, Akt isoenzyme. It can solve the problem that there is no specific PDK1 inhibitor yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] Cloning of human Akt isozyme and ΔPH-Akt1
[0221]The pS2neo vector (deposited at ATCC on April 3, 2001 under the accession number ATCCPTA-3253) was prepared as follows: the pRmHA3 vector was cut with BglII (as described in Nucl. Acid Res. 16: 1043-1061 (1988) prepared by the method), a 2734bp fragment was isolated. The pUChsneo vector (prepared as described in EMBO J. 4: 167-171 (1985)) was also cut with BglII to isolate a 4029 bp fragment. The two isolated fragments were ligated together to form a vector, designated pS2neo-1. This plasmid contains a polylinker between the metallothionein promoter and the alcohol dehydrogenase polyA addition site. It also has a neo resistance gene driven by a heat shock promoter. The pS2neo-1 vector was cut with Psp5II and BsiWI. Two complementary oligonucleotides were synthesized and then annealed (CTGCGGCCGC (SEQ. ID. NO.: 1) and GTACGCGGCCGCAG (SEQ. ID. NO.: 2)). The cut pS2neo-1 was ligated with the annealed oligonucleotides t...
Embodiment 2
[0234] Expression of human Akt isozymes and ΔPH-Akt1
[0235] Using the calcium phosphate method, the DNA containing the cloned Akt1, Akt2, Akt3 and ΔPH-Akt1 genes in the pS2neo expression vector was purified and transfected into Drosophila S2 cells (ATCC). Antibiotic (G418, 500 μg / ml) resistant cell aggregates were selected. Dilute the cells to a volume of 1.0L (approximately 7.0×10 6 / ml), adding biotin and CuSO 4 , so that the final concentrations were 50μM and 50mM. Cells were grown for 72 hours at 27°C and harvested by centrifugation. Cell pellets were stored at -70°C until use.
Embodiment 3
[0237] Purification of human Akt isoenzymes and ΔPH-Akt1
[0238] The cell pellet obtained from 1L of S2 cells described in Example 2 was dissolved in 50 ml of 1% CHAPS buffer A (50 mM Tris pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.2 mM AEBSF, 10 μg / ml benzamidine, Lysis was performed by sonication in 5 [mu]g / ml each of aprotinin, aprotinin and peptin, 10% glycerol and 1 mM DTT). The soluble fraction was purified with a protein G Sepharose (Pharmacia) column containing 9 mg / ml anti-intermediate T monoclonal antibody, and 75 μM EYMPME (SEQ.ID.NO.: 14) peptide containing 25% Glycerol was eluted in Buffer A. Fractions containing Akt were pooled and protein purity was evaluated by SDS-PAGE. Purified proteins were quantified using standard Bradford protocols. Purified proteins were snap frozen in liquid nitrogen and stored at -70°C.
[0239] Akt Purified from S2 Cells and Akt Platelet Leukocyte C Kinase Substrate Homology Domain Deletion Required for Activation Activation of Akt and Akt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com