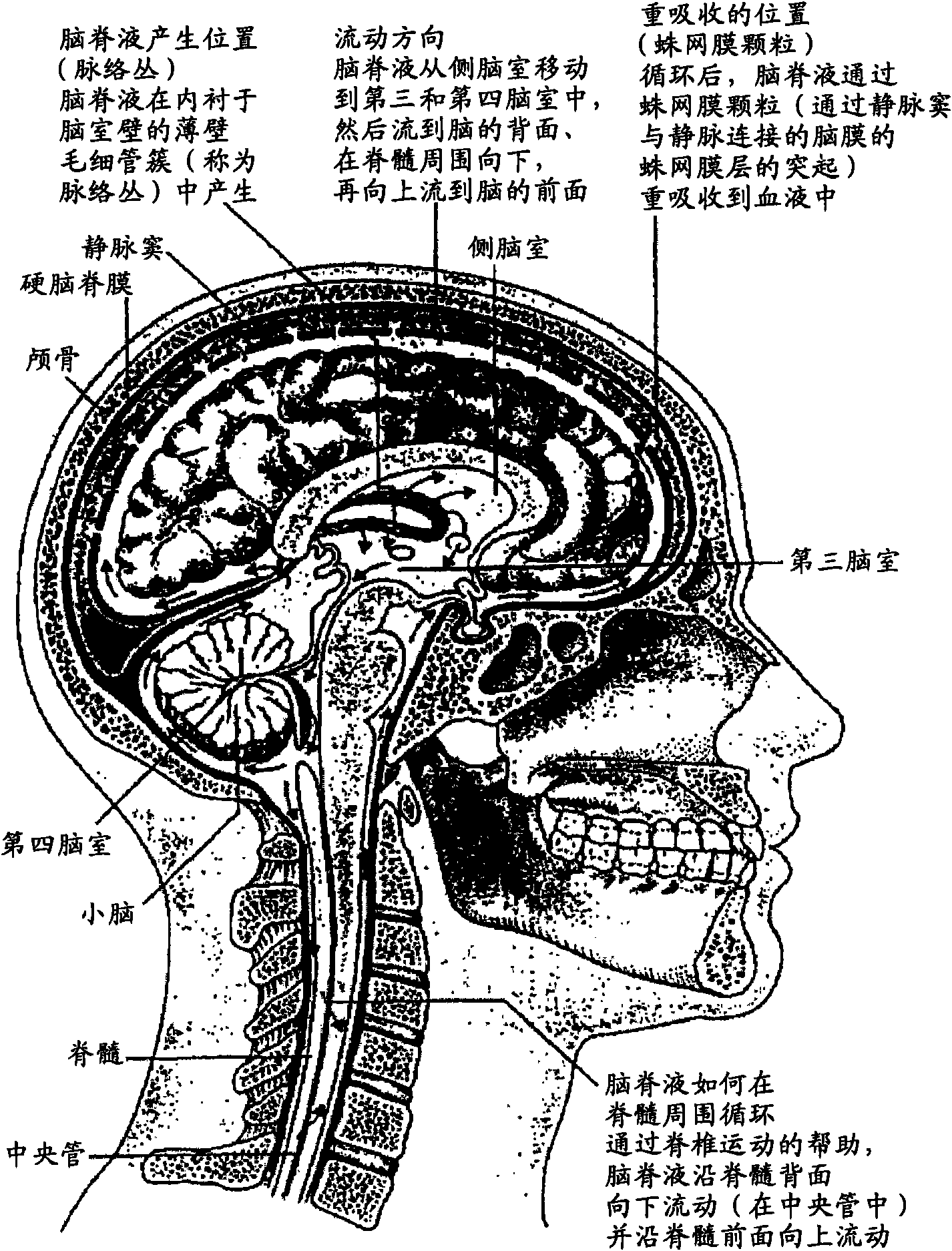
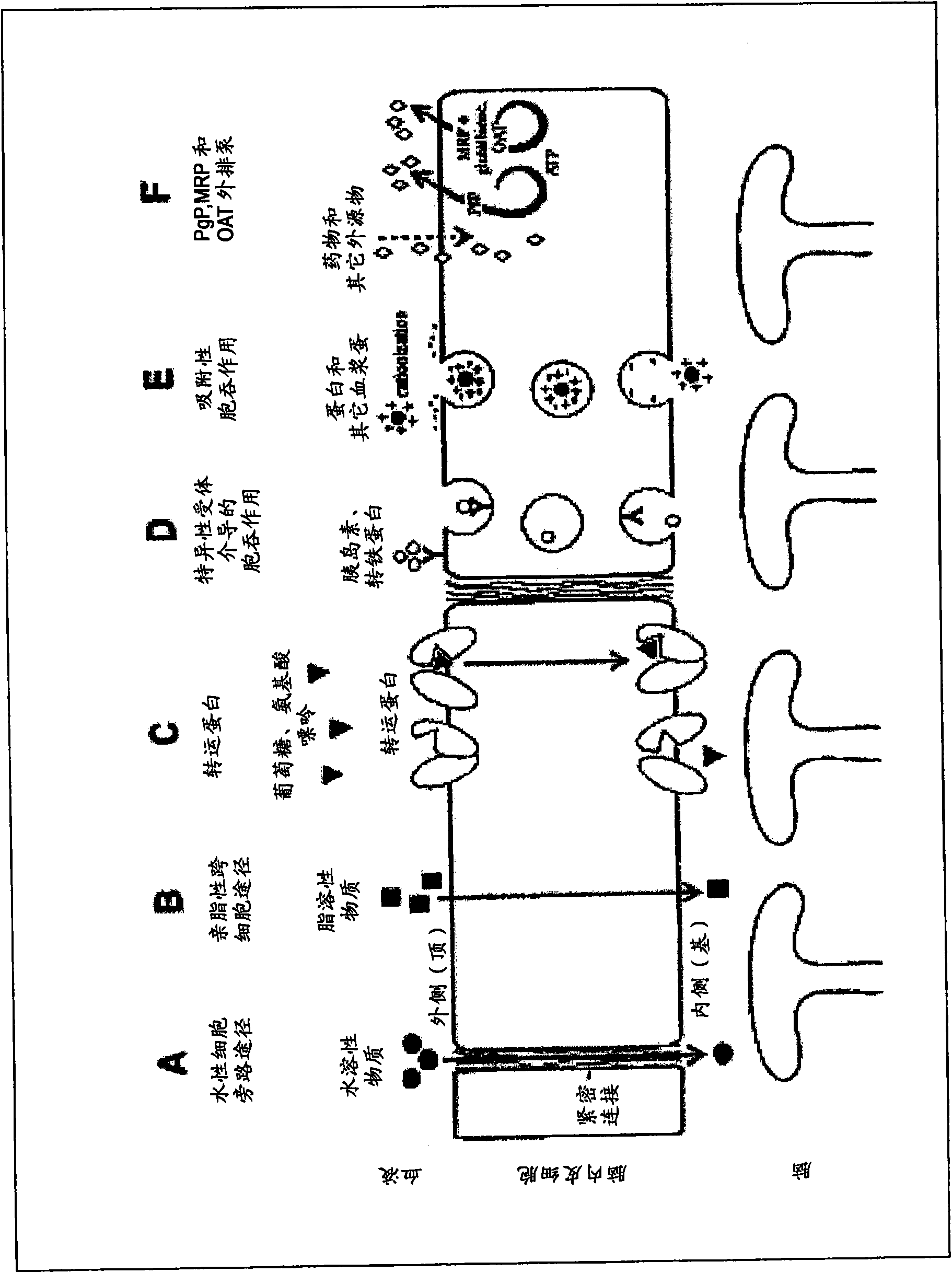
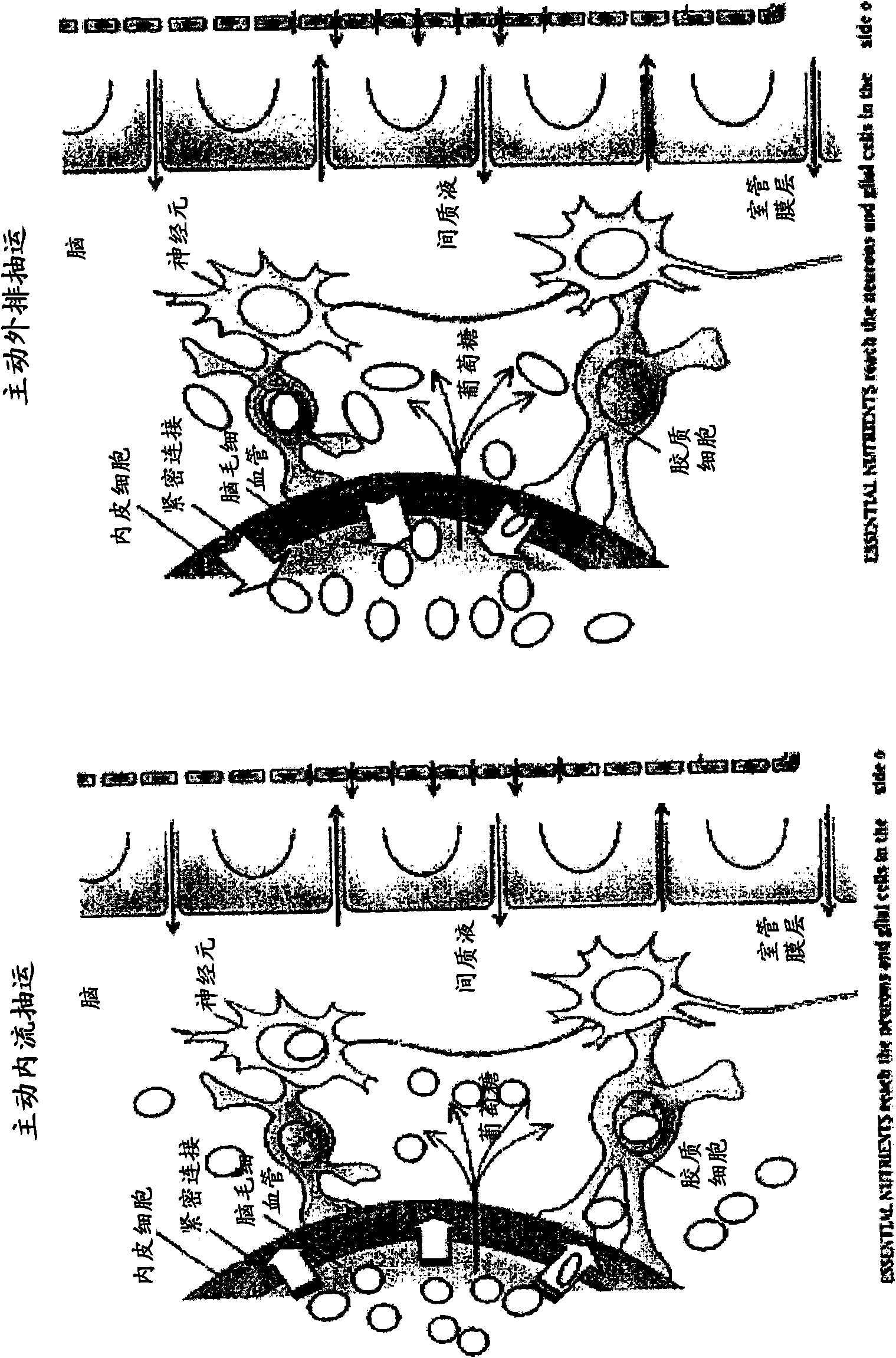
Methods and compositions for therapeutic treatment
A composition, a technique for therapeutic effect, applied in the field of neuraminidase inhibitors and sufficient to lower calcineurin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0396] Example 1: Human Study of the Effects of Quercetin (Q) and Tacrolimus on Transplant Patients
[0397] An empirical trial of the effect of oral quercetin (Q) on the CNS effects of tacrolimus can be performed. Inclusion criteria included patients who had undergone liver, kidney, and heart transplantation and who exhibited neurotoxic events (such as seizures, tremors, headache, and visual abnormalities) under tacrolimus therapy. Preferably, these patients have no prior history of prior transplantation or CNS effects associated with tacrolimus. Exemplary dosing regimens for tacrolimus are provided in the table below.
[0398]
[0399] Due to interindividual variability in the pharmacokinetics of tacrolimus, it is necessary to individualize the dosing regimen to achieve optimal therapeutic effect. The dose of tacrolimus was adjusted daily to achieve trough concentrations of 15-20 and approximately 10 ng / mL in the first and subsequent two weeks, respectively. Blood samp...
Embodiment 2
[0401] Example 2: Human study of the effect of quercetin (Q) and tacrolimus on patients with atopic dermatitis
[0402] An empirical trial of the effect of oral quercetin (Q) on the CNS effects of tacrolimus can be performed. Inclusion criteria included patients with atopic dermatitis under treatment with PROTOPIC ointment (tacrolimus) and exhibiting neurotoxic events (such as seizures, tremors, visual abnormalities, etc. . . . ). Patients can apply PROTOPIC Ointment 0.03% or PROTOPIC Ointment 0.01% to the affected skin twice a day.
[0403] 100-500 mg of Q per capsule was formulated and provided to all subjects. In some trials, placebo capsules were also formulated. Subjects were instructed to complete a daily diary for 7 days and to continue their baseline treatment and daily activities. On approximately day 7, they are asked to start administration of 2 capsules of Q (200-1000 mg) twice daily (total Q daily dose 200-2000 mg) or an equal dose of placebo, preferably in a d...
Embodiment 3
[0404] Example 3: BTB transporter activators increase the efficacy of tacrolimus
[0405] animal: 8-9 week old Lewis and Brown Norway male rats were obtained from Charles River Laboratories. The general procedures for animal care and feeding refer to the National Research Council (NRC)'s Guide for the Care and Use of Laboratory Animals (Guide for the Care and Use of Laboratory Animals) (1996) and the Animal Welfare Standards (Animal Welfare Standards).
[0406] deal with: Lewis rats were treated with different single doses of LNS 0694 i.p. 30 minutes before a single i.v. injection of tacrolimus at a concentration of 1 mg / kg as described in the table below.
[0407]
Group
LNS 0694 TM (BTB to
transport protein activator) treatment
(IP)
FK506 processing
(IV)
1
baseline
(untreated control)
---------
2
50mg / kg
1mg / kg
3
150mg / kg
1mg / kg
4
300mg / kg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com