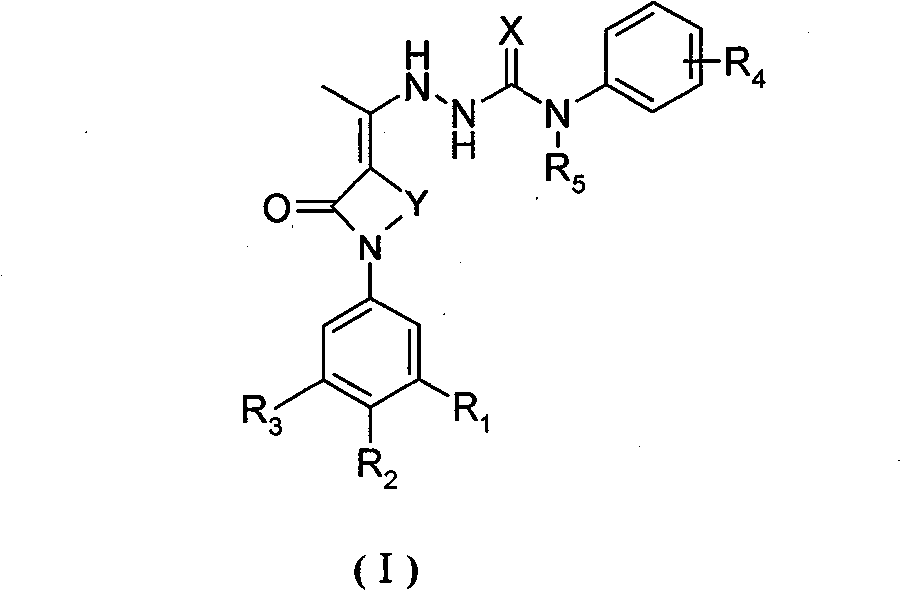
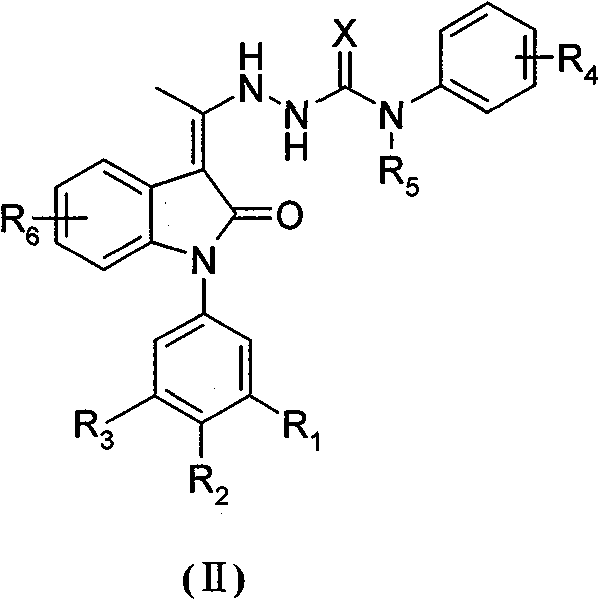
Ethylidene hydrazine carboxamide derivatives, preparation process and pharmaceutical use thereof
An alkyl and pharmacy technology, applied in the direction of pharmaceutical formulations, drug combinations, medical preparations containing active ingredients, etc., can solve the problem of lack of natural TPO sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103]
[0104] first step
[0105] Methyl 3-(hydrazinesulfonylamino)benzoate
[0106] Dissolve methyl 3-isothiocyanate benzoate 1a (3.0g, 15.5mmol) in 30mL of absolute ethanol, add 50% hydrazine hydrate solution (3mL, 30mmol) and 20mL of absolute ethanol while stirring, and heat to reflux 1 hour. The reaction was tracked by TLC until the raw materials disappeared, the reaction solution was cooled to room temperature, filtered, and the filter cake was dried under vacuum to obtain methyl 3-(hydrazinesulfonylamino)benzoate 1b (3.1 g, white solid). Yield: 88.8%.
[0107] MSm / z(ESI): 226[M+1]
[0108] 1 HNMR (400MHz, CD 3 OD-d 4): δ9.25(s, 1H), 8.35(s, 1H), 7.88(d, 1H, J=6.4Hz), 7.69(d, 1H, J=7.2Hz), 7.44(t, 1H, J= 8.4Hz), 4.76(br, 2H), 3.86(s, 3H)
[0109] second step
[0110] 1-m-tolyl-1,3-dihydroindolin-2-one
[0111] Using a known method [Bulletinde la Societe Chimique de France (1968), (3), 1090-1.], 1,3-dihydroindolin-2-one 1c (20g, 150mmol), m-bromotoluene (30....
Embodiment 2
[0130]
[0131] first step
[0132] 1-(3,4-Xylyl)-1,3-dihydroindolin-2-one
[0133] 1,3-indolin-2-one 2a (8.0g, 60.1mmol), 4-bromo-1,2-xylene (13.4g, 72.2mmol), cuprous iodide (2.29g, 12.0mmol ) and potassium carbonate (20.7g, 150mmol) were dissolved in 250mL of acetonitrile, N, N'-dimethyl-1,2-ethylenediamine (1.59g, 18mmol) was added under stirring, and heated to reflux for 2 hours. TLC followed the reaction until the raw materials disappeared, added 500mL of water, adjusted the pH=5 with 1N hydrochloric acid, extracted with ethyl acetate (100mL×3), dried over anhydrous magnesium sulfate, filtered, concentrated the filtrate under reduced pressure, and purified the obtained product by silica gel column chromatography The residue gave 1-(3,4-xylyl)-1,3-indolin-2-one 2b (8.0 g, yellow solid). Yield: 57.1%.
[0134] MSm / z(ESI): 238[M+1]
[0135] second step
[0136] (Z)-3-(1-Dimethylaminoethylene)-1-(3,4-xylyl)-1,3-dihydroindolin-2-one
[0137] Dissolve 1-(3,4-xylyl)-1,...
Embodiment 3
[0157]
[0158] first step
[0159] 1-(4-Methylphenyl)-1,3-dihydroindolin-2-one
[0160] Using a known method [Bulletinde la Societe Chimique de France (1968), (3), 1090-1.], 1,3-dihydroindolin-2-one 1c (13.3g, 100mmol), p-bromotoluene (20.5g, 120mmol) , cuprous iodide (3.8g, 20mmol) and potassium carbonate (30.36g, 220mmol) were dissolved in 150mL acetonitrile, and N, N'-dimethyl-1,2-ethylenediamine (2.67g, 30mmol) was added under stirring ), heated to reflux for 2 hours. TLC followed the reaction until the raw materials disappeared, added 500mL of water, adjusted the pH=5 with 1N hydrochloric acid, extracted with ethyl acetate (150mL×3), dried over anhydrous magnesium sulfate, filtered, the filtrate was concentrated under reduced pressure, and purified by silica gel column chromatography The residue gave the title product 1-(4-methylphenyl)-1,3-indolin-2-one 3a (16.0 g, pale yellow solid). Yield: 71.7%.
[0161] MSm / z(ESI): 224[M+1]
[0162] second step
[0163] (Z)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com