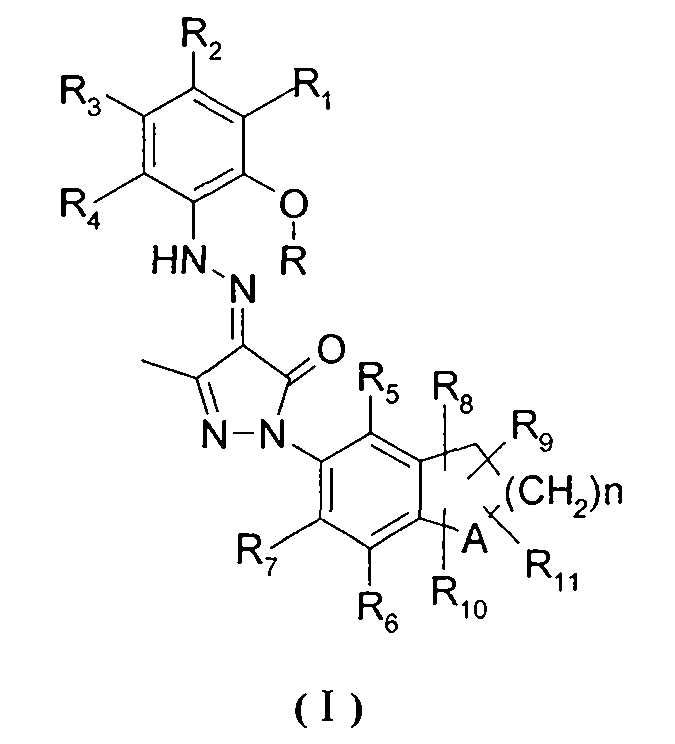
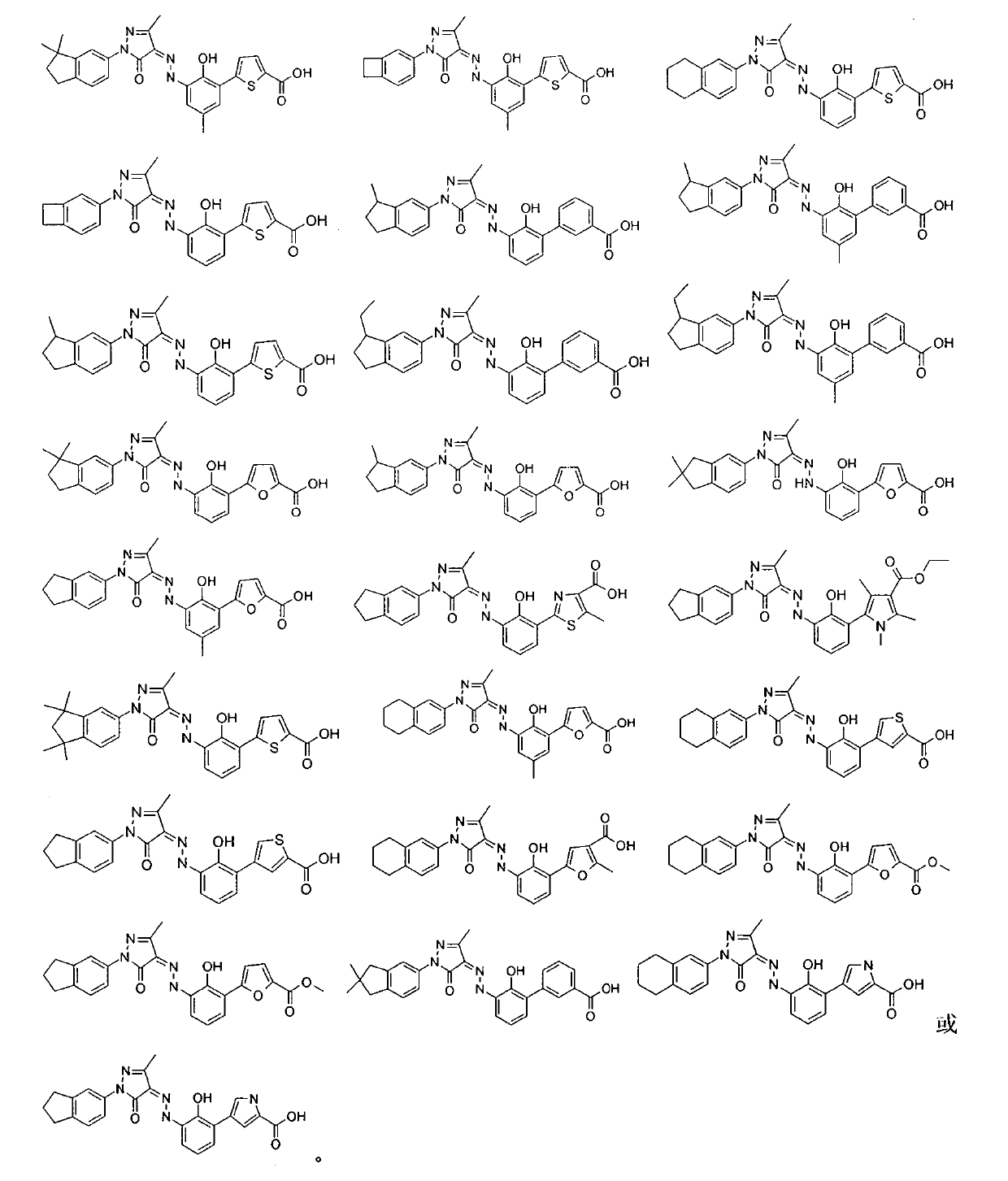Bicyclo-substituted pyrazolon azo derivatives, preparation process and pharmaceutical use thereof
A pharmacy and compound technology, applied in the field of bicyclic substituted pyrazolone azo derivatives, can solve problems such as lack of natural TPO sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] 2'-Hydroxy-3'-[N'-(1-indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazole-4-ylidene)-hydrazino] -Biphenyl-3-carboxylic acid
[0093]
[0094] first step
[0095] 2-Bromo-6-nitrophenol
[0096]Dilute 60 mL of concentrated sulfuric acid into 186 mL of water, add sodium nitrate (79.2 g, 0.932 mol) after cooling to room temperature, keep below 25 °C, add o-bromophenol 1a (60 mL, 0.516 mol) dropwise, and react at room temperature for 2 hours. TLC tracked until the raw material disappeared, and added 320mL ethyl acetate to dissolve the separated solid, washed with water and saturated sodium chloride solution, dried with anhydrous magnesium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the obtained residue was purified by silica gel column chromatography. The title product 2-bromo-6-nitrophenol 1b was obtained (48.2 g, yellow solid). Yield: 42.8%.
[0097] MS m / z(ESI): 218[M+1]
[0098] 1 HNMR (400MHz, CDCl 3 ): δ6.88-7.02(m, 1H), 7.89-7.91(...
Embodiment 2
[0133] 5'-fluoro-2'-hydroxy-3'[N'-(1-indan-5-yl-3-methyl-5-oxo-1,5-dihydropyrazole-4-ylidene) -hydrazino]-biphenyl-3-carboxylic acid
[0134]
[0135] first step
[0136] 2-Bromo-4-fluoro-6-nitrophenol
[0137] 2-Bromo-4-fluoro-phenol 2a (8.0g, 41.9mmol) was dissolved in 10mL sulfuric acid solution (50%) under ice-salt bath, and sodium nitrate (7.1g, 83.5mmol) was added dropwise in 24mL sulfuric acid solution (25 %), reacted at room temperature for 1.5 hours. TLC tracked until the raw material disappeared, added 50mL of water, extracted with ethyl acetate (50mL×2), combined the organic phases, washed the ethyl acetate layer with water and saturated sodium bicarbonate solution, dried with anhydrous magnesium sulfate, filtered, and the filtrate was reduced to Concentrated under reduced pressure to obtain the title product 2-bromo-4-fluoro-6-nitrophenol 2b (8.0 g, red solid), which was directly used in the next reaction, yield: 80.8%.
[0138] second step
[0139] 1-Bro...
Embodiment 3
[0161] 2'-Hydroxy-3'-{N'-[3-methyl-5-oxo-1-(5,6,7,8-tetrahydronaphthalene-2-yl)-1,5-dihydropyridine Azol-4-ylidene]-hydrazino}-biphenyl-3-carboxylic acid
[0162]
[0163] first step
[0164] 2-hydrazino-5,6,7,8-tetrahydronaphthalene
[0165] Dissolve 3 g (3.68 g, 25.0 mmol) of 2-amino-5,6,7,8-tetrahydronaphthalene in 20 mL of concentrated hydrochloric acid under an ice bath, and stir for 10 minutes. 10 mL of sodium nitrite solution (1.72 g, 25.0 mmol) was added dropwise, and stirring was continued for 15 minutes under ice-cooling.
[0166] Dissolve stannous chloride dihydrate (22.6 g, 100 mmol) in 10 mL of concentrated hydrochloric acid in an ice-salt bath, add the spare intermediate solution, and react at room temperature for 1.5 hours. Adjust the pH to 9 with 40% sodium hydroxide solution in an ice bath, add 400 mL of ethyl acetate to extract, concentrate under reduced pressure, and dry to obtain the title product 2-hydrazino-5,6,7,8-tetrahydronaphthalene 3h (2.19 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com