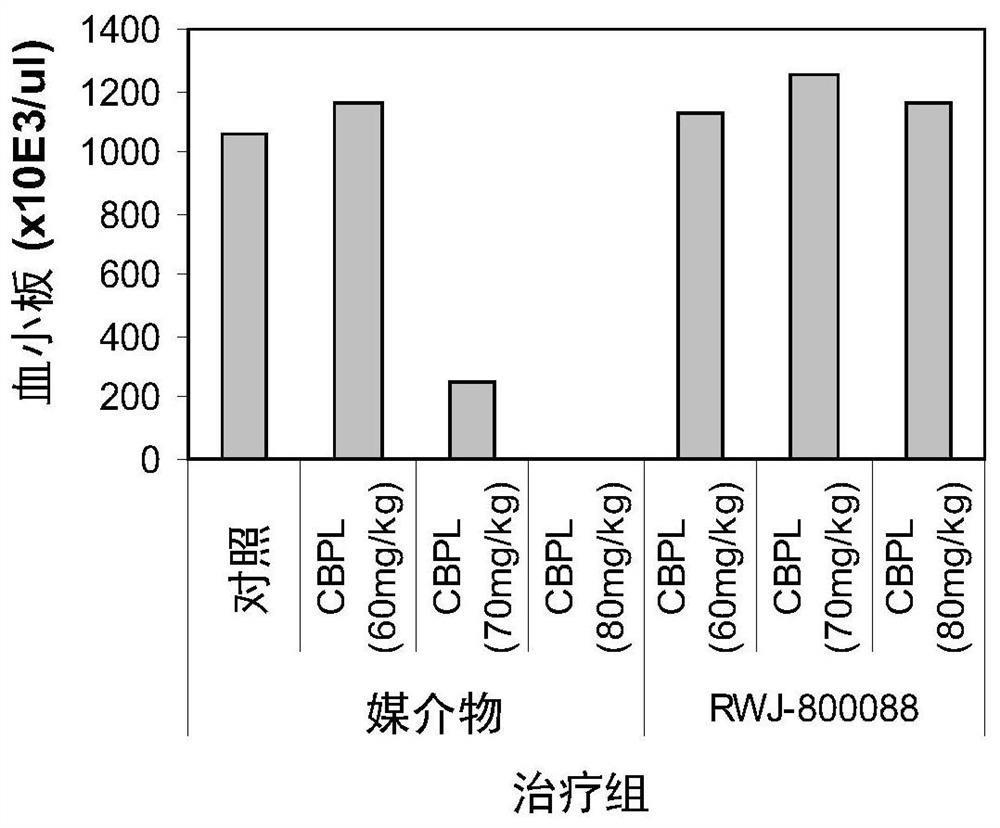
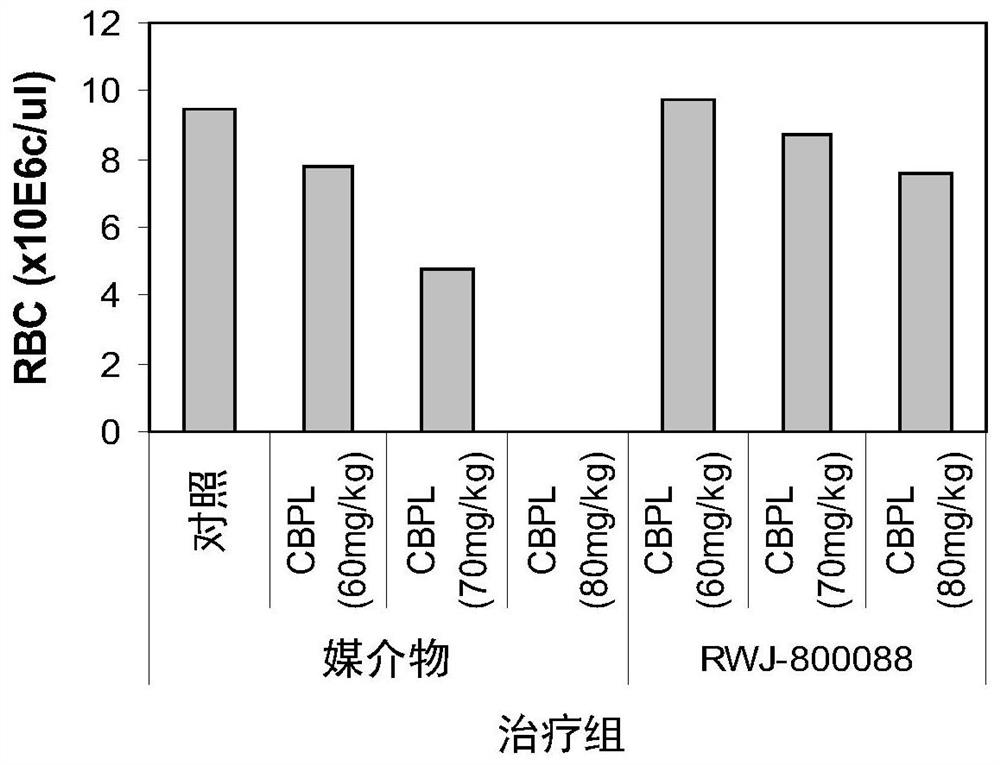
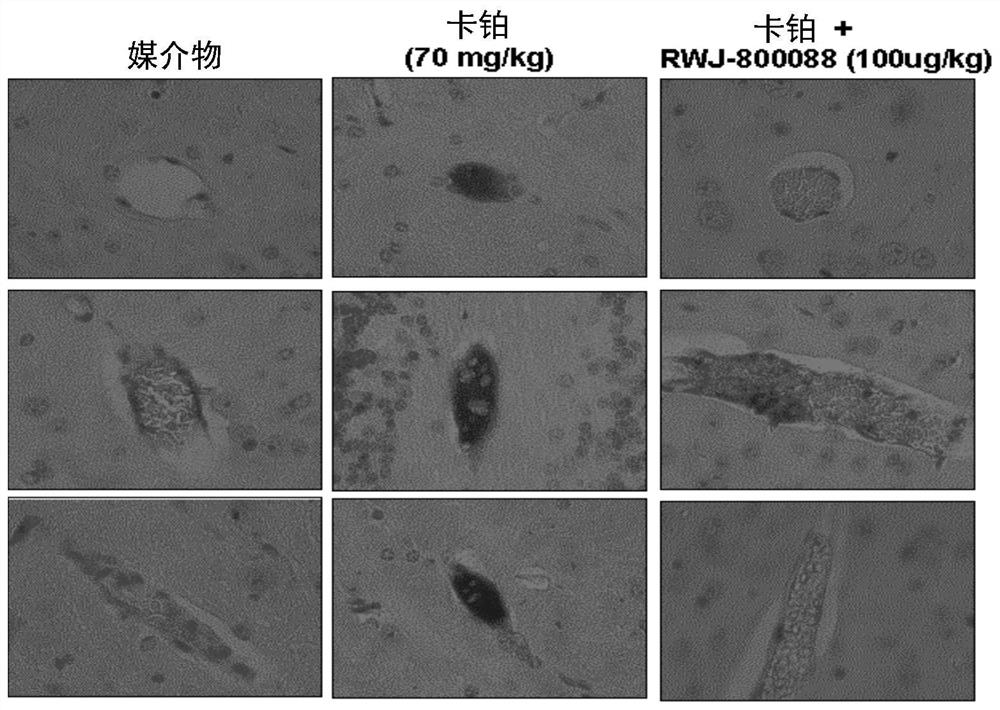
Methods of enhancing protection against organ and vascular injury, hematopoietic recovery and survival in response to total body radiation/chemical exposure
A technology for vascular injury and chemotherapy, which can be used in pharmaceutical formulations, peptide/protein components, medical preparations with non-active ingredients, etc., and can solve problems such as serious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0133] Embodiment 1(a) is a method of reducing vascular injury in a human subject treated with radiation therapy or chemotherapy, the method comprising administering to the human subject in need thereof an effective amount of thrombopoietin (TPO) mimetic, wherein said TPO mimetic comprises the amino acid sequence of SEQ ID NO: 1, and said effective amount comprises 0.1 microgram (μg) to 6 μg of TPO mimetic per kilogram (kg) of subject body weight, or Fixed or stratified dose equivalents based on typical body weight for the subject population.
[0134] Embodiment 1(b) is a method of promoting organ and / or hematopoietic recovery in a human subject treated with radiation therapy or chemotherapy, the method comprising administering to the human subject in need thereof an effective amount of A thrombopoietin (TPO) mimic, wherein the TPO mimic comprises the amino acid sequence of SEQ ID NO: 1, and the effective amount comprises 0.1 microgram (μg) to 6 μg of the TPO mimic per kilogra...
Embodiment approach 2
[0150] Embodiment 2(h) is the method of embodiment 2, wherein the TPO mimetic is administered to the subject from about 0 minutes to about 32 hours prior to treating the subject with radiation therapy or chemotherapy.
[0151] Embodiment 2(i) is the method of embodiment 2(h), wherein the TPO mimetic is administered to the subject about 10 minutes to about 28 hours before the subject is exposed to radiation therapy or chemotherapy thing.
[0152] Embodiment 2(j) is the method of embodiment 2(h), wherein the TPO mimetic is administered to the subject about 10 minutes to about 24 hours before the subject is exposed to radiation therapy or chemotherapy things.
[0153] Embodiment 2(k) is the method of embodiment 2(h), wherein the TPO mimetic is administered to the subject about 10 minutes to about 20 hours before the subject is exposed to radiation therapy or chemotherapy thing.
[0154] Embodiment 2(l) is the method of embodiment 2(h), wherein the TPO mimetic is administered t...
Embodiment approach 3
[0159] Embodiment 3(a) is a method of reducing vascular injury in a subject treated with radiation therapy or chemotherapy, the method comprising administering to the subject in need thereof an effective amount of thrombopoietin (TPO ) mimetic, wherein the TPO mimetic comprises the amino acid sequence of SEQ ID NO: 1, and is administered to the subject within about 32 hours prior to treating the subject with at least one of radiation therapy and radiomimic chemotherapy The TPO mimetic is administered to the patient.
[0160] Embodiment 3(b) is a method of promoting organ and / or hematopoietic recovery in a subject treated with radiation therapy or chemotherapy, the method comprising administering to the subject in need thereof an effective amount of platelets TPO mimetic, and administering the TPO mimetic to the subject within about 32 hours prior to treating the subject with at least one of radiation therapy and radiation-mimicking chemotherapy.
[0161] Embodiment 3(c) is a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com