Methods for mitigating liver injury and promoting liver hypertrophy, regeneration and cell engraftment in conjunction with radiation and/or radiomimetic treatments
A technology of radiation therapy and mimics, applied in the field of reducing liver damage and promoting liver hypertrophy, regeneration and cell implantation combined with radiation and/or radiation mimic therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
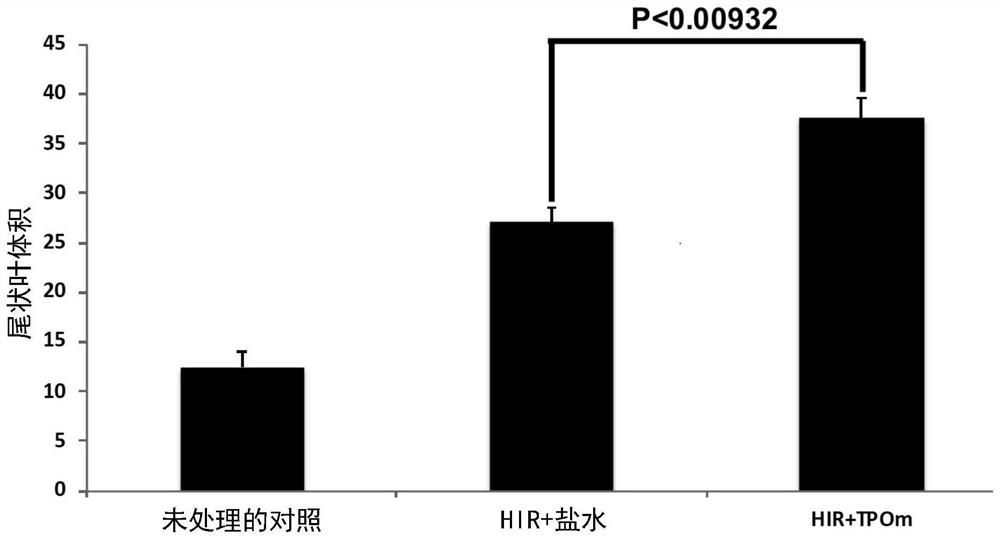
[0130] Embodiment 1 is a method of alleviating radiation-induced liver disease (RILD) in a subject in need thereof, the method comprising administering to the subject an effective amount of a thrombopoietin (TPO) mimetic, wherein Administering to the subject an effective amount of the TPO mimetic reduces radiation-induced liver disease in the subject.
[0131] Embodiment 1(a) is the method of embodiment 1, wherein the TPO mimetic comprises a peptide having the amino acid sequence of SEQ ID NO:1.
[0132] Embodiment 1(b) is the method of embodiment 1(a), wherein the peptide has the amino acid sequence of SEQ ID NO:2.
[0133] Embodiment 1(c) is the method of Embodiment 1(a) or 1(b), wherein the TPO mimetic further comprises a hydrophilic polymer covalently linked to the peptide.
[0134] Embodiment 1(d) is the method of Embodiment 1(c), wherein the hydrophilic polymer is any of: i) polyethylene glycol (PEG), ii) polypropylene glycol, iii) polylactic acid, or iv) Polyglycolic ...
Embodiment approach 2
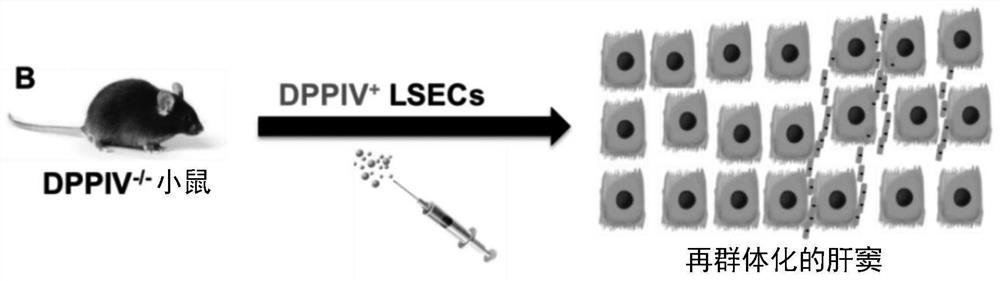
[0145] Embodiment 2 is the method of any one of embodiments 1-1(m)(1), wherein the radiation-induced liver disease is any one or more of the following: hepatomegaly, hepatic necrosis, apoptosis Death, ascites, elevated liver enzymes, thrombocytopenia, sinusoidal obstruction syndrome (SOS) and central veno-occlusive disease (VOD).
[0146] Embodiment 2(a) is the method of Embodiment 2, wherein the radiation-induced liver disease is hepatomegaly.
[0147] Embodiment 2(b) is the method of Embodiment 2, wherein the radiation-induced liver disease is ascites.
[0148] Embodiment 2(c) is the method of Embodiment 2, wherein the radiation-induced liver disease is elevated liver enzymes.
[0149] Embodiment 2(d) is the method of Embodiment 2, wherein the radiation-induced liver disease is thrombocytopenia.
[0150] Embodiment 2(e) is the method of Embodiment 2, wherein the radiation-induced liver disease is sinusoidal obstruction syndrome (SOS).
[0151] Embodiment 2(f) is the metho...
Embodiment approach 3
[0157] Embodiment 3(c) is the method of Embodiment 3(a) or 1(b), wherein the TPO mimetic further comprises a hydrophilic polymer covalently linked to the peptide.
[0158] Embodiment 3(d) is the method of Embodiment 3(c), wherein the hydrophilic polymer is any of: i) polyethylene glycol (PEG), ii) polypropylene glycol, iii) polylactic acid, or iv) Polyglycolic acid.
[0159] Embodiment 3(e) is the method of Embodiment 3(d), wherein the hydrophilic polymer is PEG.
[0160] Embodiment 3(f) is the method of embodiment 3(e), wherein the PEG is monomethoxypolyethylene glycol (MePEG-OH), monomethoxypolyethylene glycol-succinate (MePEG-OH), S), monomethoxypolyethylene glycol-succinimidyl succinate (MePEG-S-NHS), monomethoxypolyethylene glycol-amine (MePEG-NH2), monomethoxypolyethylene glycol Either of diol-trifluoroethanesulfonate (MePEG-TRES) or monomethoxypolyethylene glycol-imidazolyl-carbonyl (MePEG-IM).
[0161] Embodiment 3(g) is the method of Embodiment 3(e), wherein the PE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com