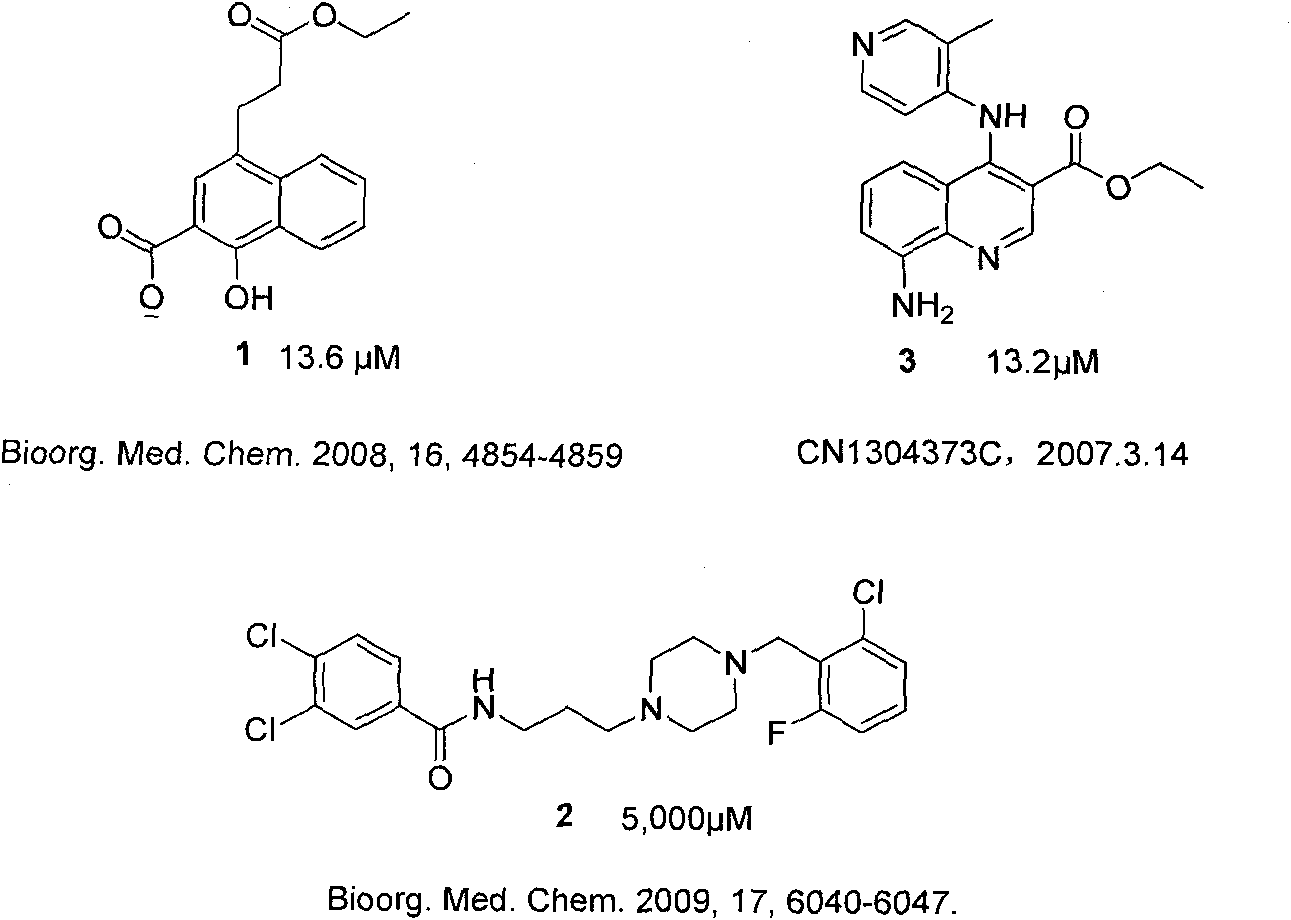
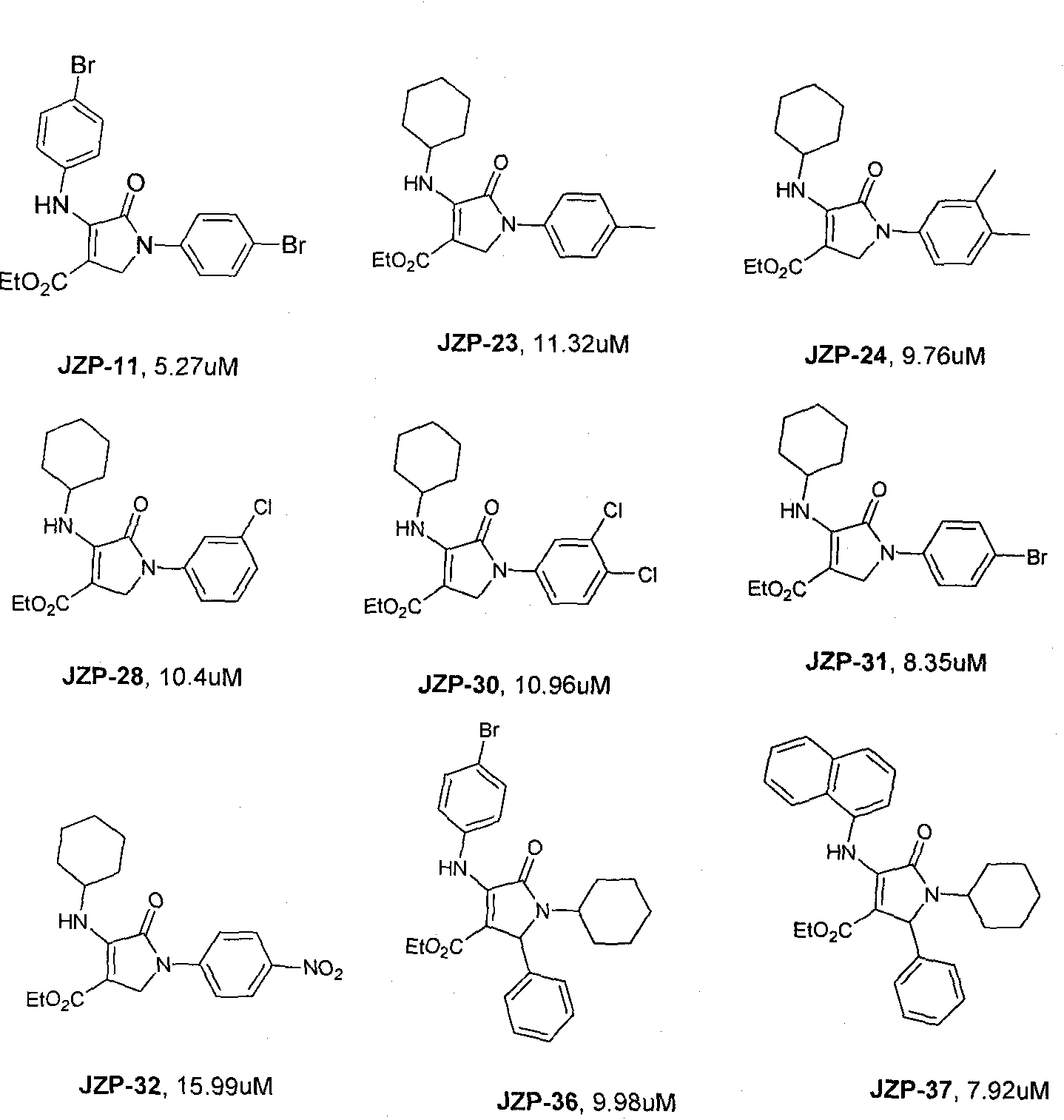
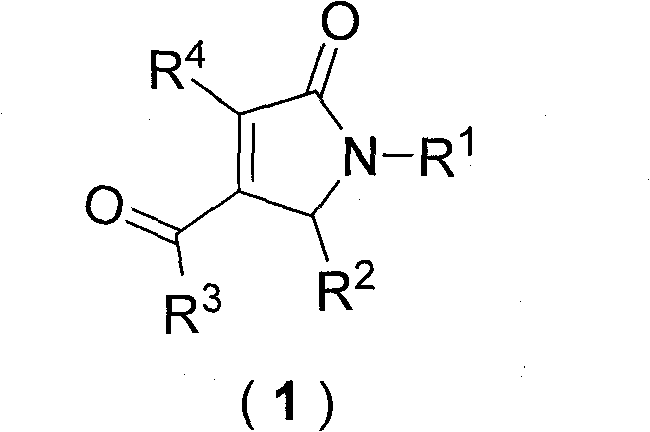
Dihydropyrrolones derivative as Caspase-3 inhibitor
A technology of dihydropyrrolidone and its derivatives, which can be used in anti-inflammatory agents, antiviral agents, drug combinations, etc., and can solve problems such as cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] For the synthesis method in Example 1, refer to Method B reported in the literature (J.Comb.Chem.2009,11,685-696): primary aromatic amine (4mmol), 38% formaldehyde (120mg, 1.5mmol) and acetic acid ( 120mg, 2mmol) were sequentially added to the reaction solution (reaction 10-30 minutes) of the primary amine arylamine (1mmol) and butyndioic acid diester (1mmol) with ethanol (4mL) as the solvent, and stirred at 70°C until the reaction was complete (Reaction monitored by TLC). Use 20×20 GF 254 A thin-layer chromatography plate uses a mixed solvent of petroleum ether: ethyl acetate = 10:1-1:1 as a developing solvent, and uses ethyl acetate as an eluent to separate and purify the product.
[0051] For the synthesis method in Examples 2-15, refer to Method A reported in the literature (J.Comb.Chem.2009, 11, 685-696): primary amine (2mmol), 38% formaldehyde (120mg, 1.5mmol) and acetic acid (120mg, 2mmol) was added successively to the reaction solution (reaction of 10-30 minut...
Embodiment 2
[0056] 2,5-Dihydro-1-n-propyl-4-(cyclohexylamino)-5-oxo-1H-pyrrole-3-carboxylic acid ethyl ester (ethyl4-(cyclohexylamino)-2,5-dihydro-5- oxo-1-propyl-1H-pyrrole-3-carboxylate compound number JZP-20 / 749-1). Yellowish solid, 1H NMR (600MHz, CDCl3): δ=6.75(b, 1H), 4.595-4.591(m, 1H), 4.20(q, J=7.2Hz, 2H), 3.93(s, 2H), 3.41 (t, J=7.2Hz, 2H), 1.98-1.96(m, 2H), 1.73-1.67(m, 11H), 0.92(t, J=7.2Hz, 3H) ppm.13C NMR (150MHz, CDCl3): δ = 165.4, 100.0, 59.4, 44.5, 34.9, 25.5, 24.7, 21.5, 14.6, 11.3 ppm. GC-MS: 294 (M+).
Embodiment 3
[0058] 2,5-Dihydro-1-cyclohexyl-4-(cyclohexylamino)-5-oxo-1H-pyrrole-3-carboxylic acid ethyl ester (ethyl1-cyclohexyl-4-(cyclohexylamino)-2,5-dihydro - 5-oxo-1H-pyrrole-3-carboxylate Compound No. JZP-21 / 749-2). White solid; 1H NMR (600MHz, CDCl3): δ=6.62(b, 1H), 4.48(b, 1H), 4.09-4.08(m, 2H), 3.89(b, 1H), 3.80(b, 2H), 1.87-1.28 (m, 10H), 1.19-1.05 (m, 13H) ppm.13CNMR (150MHz, CDCl3): δ=165.6, 164.7, 96.4, 59.2, 51.1, 50.3, 43.4, 34.8, 31.0, 25.5, 25.4, 24.7, 14.5 ppm. GC-MS: 334 (M+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com