Transdermally absorptive preparation
A technology of patch and preparation, applied in the field of transdermal absorption patch, can solve problems such as research and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10、12
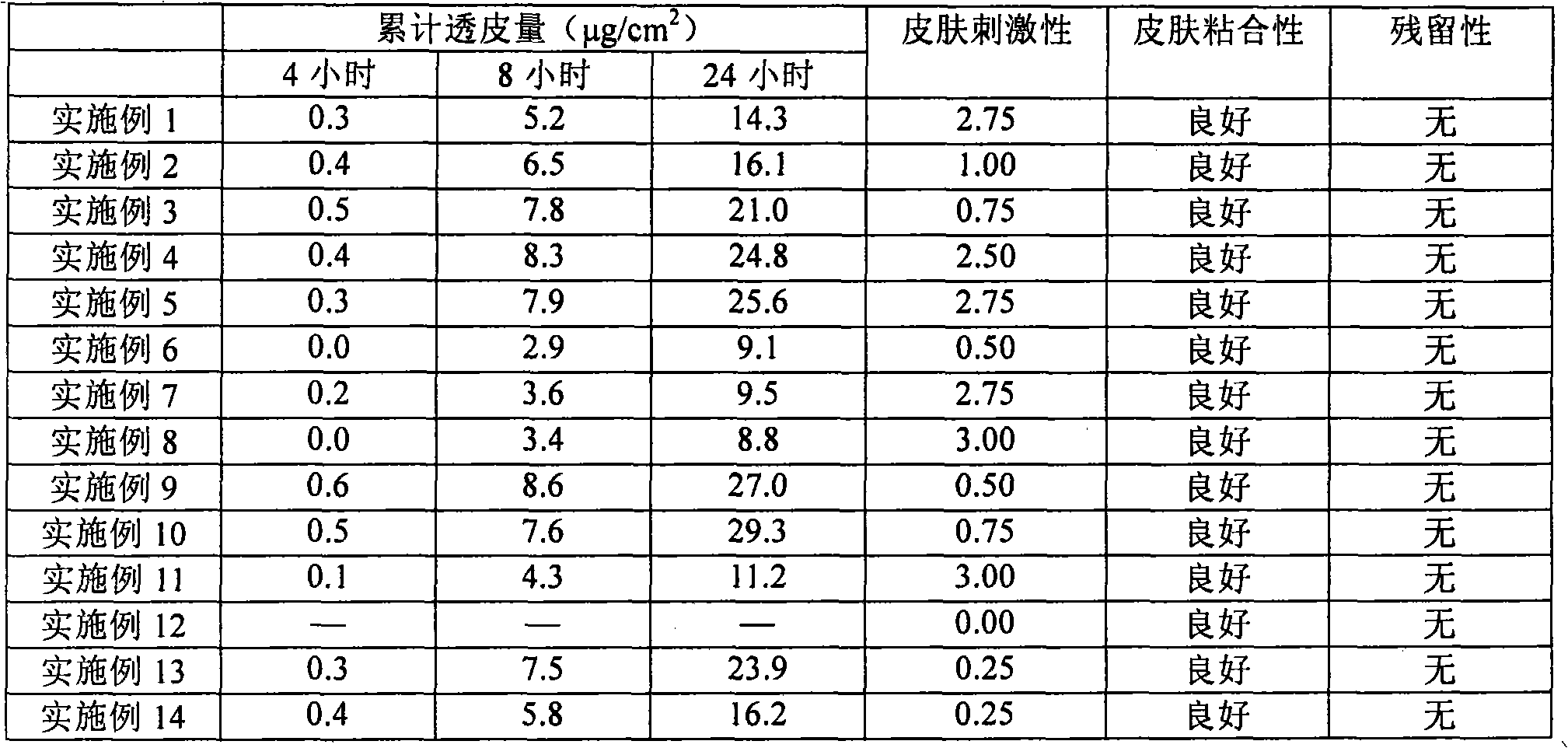
[0062] According to the ratio (weight ratio) shown in Table 1, after the binder A-D solution, glycerin, cholesterol, sodium lauryl sulfate and glyceryl monocaprate are mixed, add according to the ratio (weight ratio) shown in Table 1 Alendronate, incadronate, and PEG-modified alendronate dissolved in 20 parts by weight of water are then coated on a carbamate film with a thickness of 40 μm, and after drying, a film with a thickness of 200 μm of the adhesive layer is obtained. Patches for transdermal absorption.
Embodiment 11
[0064] Take 32 g of ethylene-isoprene-ethylene copolymer (manufactured by Kraton Japan, trade name [Kraton D1107]), alicyclic hydrocarbon tackifier (manufactured by Arakawa Chemical Co., trade name [Arkon P-90]) and liquid 28 g of paraffin wax (manufactured by Nacarai Desk, Chemical Reagent) was put into a mixer, and mixed at 120° C. to prepare a hot-melt adhesive (adhesive E). Lower the temperature to 90°C, add 2 g of sodium lauryl sulfate and 10 g of alendronate and mix well, then coat and dry the film on a polyethylene terephthalate film to obtain a patch with an adhesive layer thickness of 200 μm percutaneous absorption preparations.
[0065] [Table 1]
[0066] Example
[0067] Adhesives A-D are by solid content weight
Embodiment 13,14
[0069] After binder A or B, glycerin or sodium lauryl sulfate and glyceryl monocaprate are mixed by the proportioning (weight ratio) shown in Table 1, add and dissolve in the proportioning (weight ratio) shown in Table 1 Alendronate in 20% water by weight, then add dibutyl hydroxytoluene or vitamin E according to the proportion (weight ratio) shown in Table 1 and mix, then, coat a carbamate film with a thickness of 40 μm, and dry it to prepare A patch type percutaneous absorption preparation having an adhesive layer thickness of 200 μm was obtained.
[0070] The transdermal drug absorption test was carried out using the prepared patch type transdermal absorption preparation, and the results are shown in Table 2. The drug percutaneous absorption test was carried out as follows.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com