Small molecule leptin receptor modulators
A solvate, tautomer technology, applied in the field of small molecule leptin receptor modulators, can solve problems such as reducing food intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
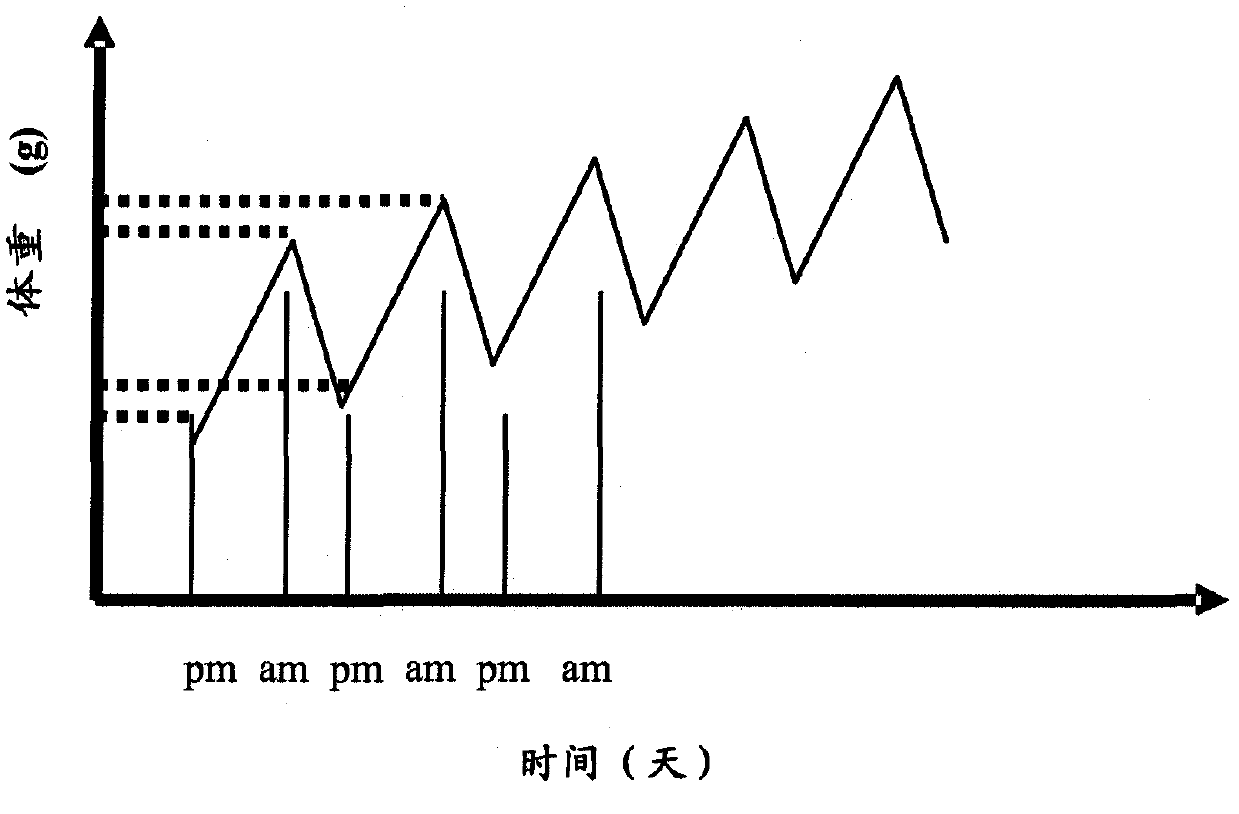
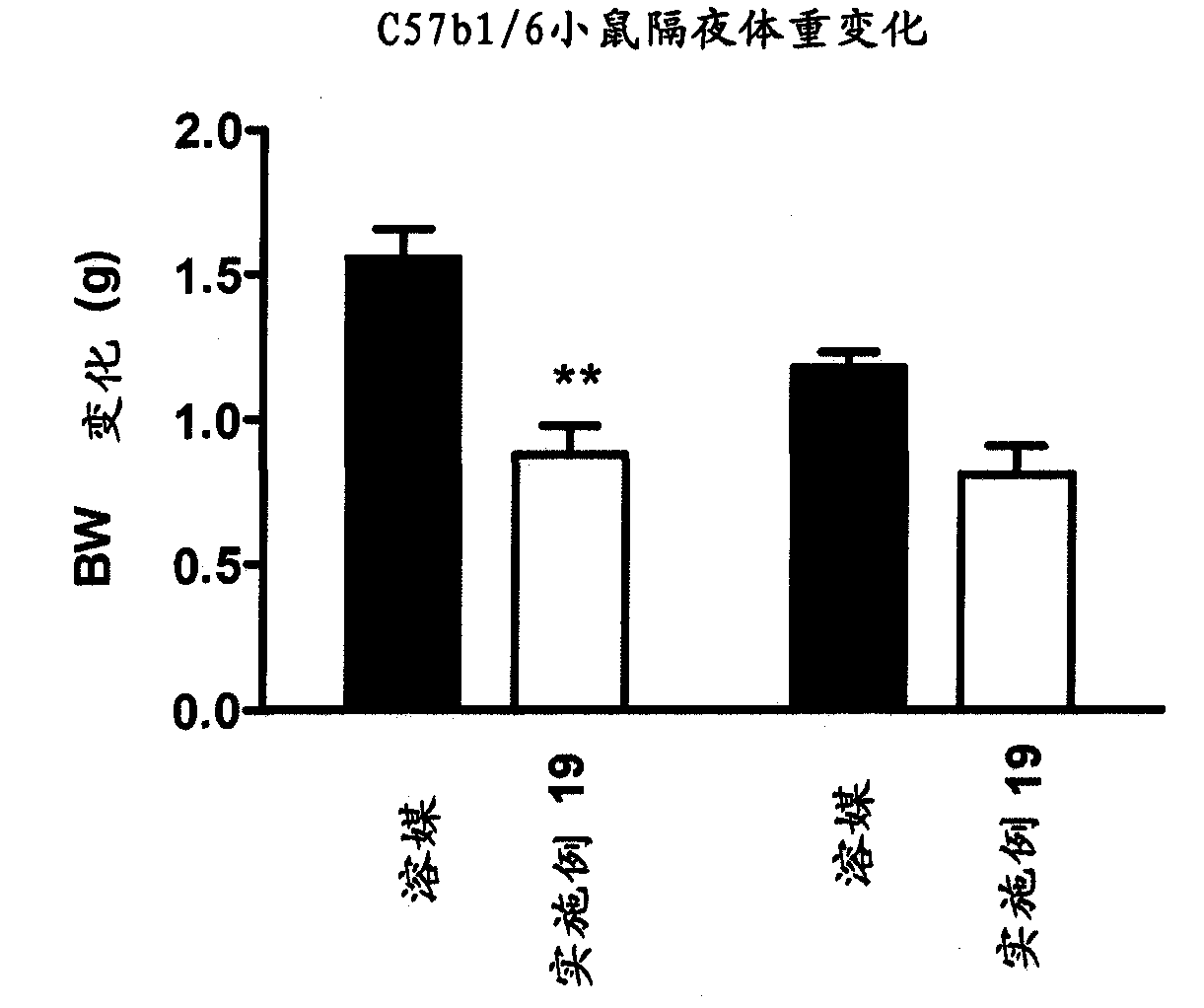
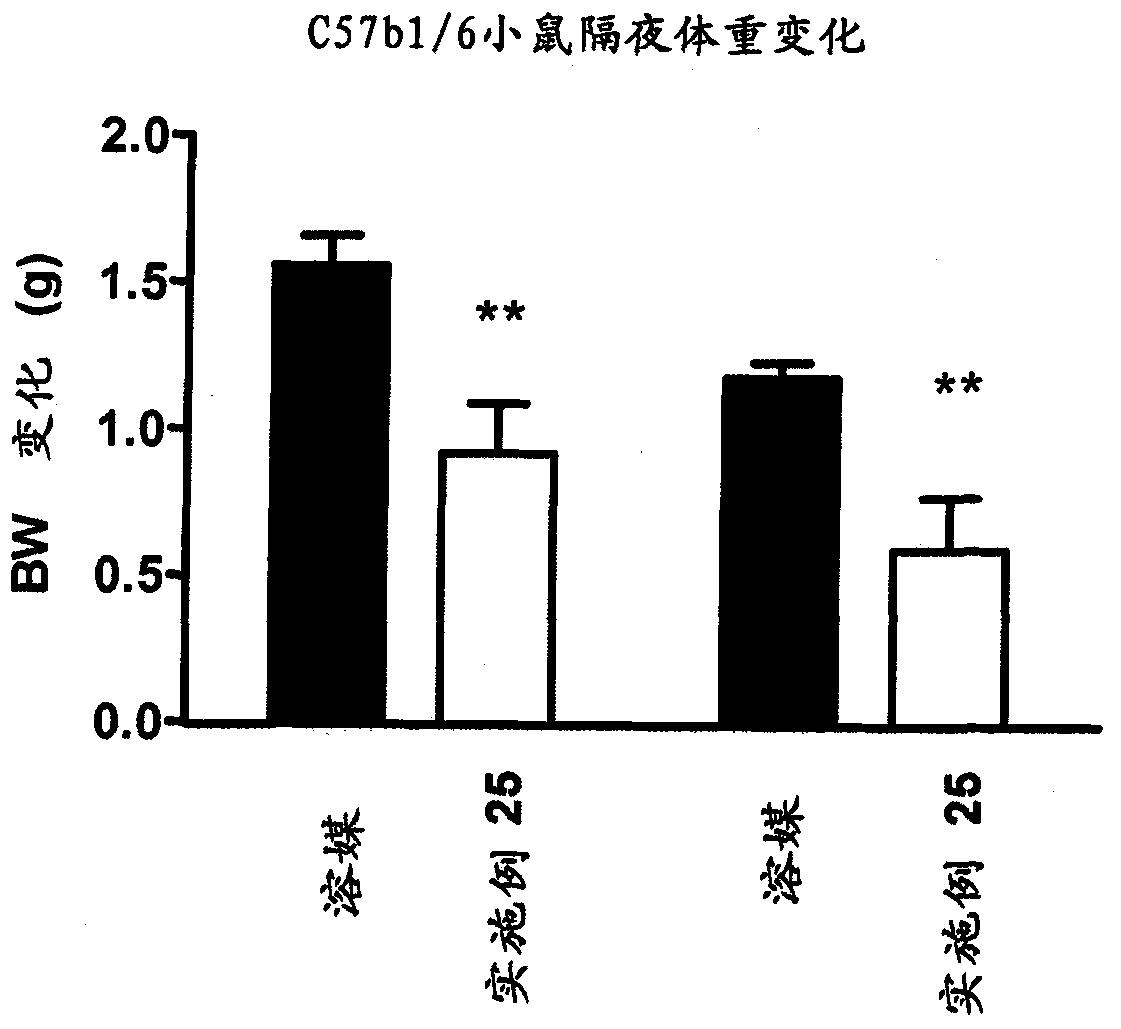
Image
Examples
Embodiment 1
[0234] 2-Piperazin-1-ylethyl {(1S)-1-(4-hydroxybenzyl)-2-[methyl(3-methylbutyl)amino]-2-oxoethyl}carbamate Dihydrochloride
[0235]
[0236] (S)-1-(carboxy)-2-(4-tert-butoxyphenyl)ethylcarbamate 2-(4-(tert-butoxycarbonyl)piperazin-1-yl)ethyl ester (intermediate Enzyme 1; 415 mg, 0.84 mmol), N-methylisoamylamine (85 mg, 0.84 mmol) and DIPEA (0.40 mL, 2.30 mmol) were dissolved in DMF (10 mL), then cooled in an ice-water bath. PyBrOP (400 mg, 0.86 mmol) was added. The reaction mixture was stirred at 0 °C for 6 hours, then allowed to warm to room temperature overnight. The reaction mixture was concentrated in vacuo. The residue was suspended in 0.2M aqueous HCl (50 mL) and extracted with DCM (3 x 50 mL). The combined DCM extracts were dried (MgSO 4 ), concentrated in vacuo and purified by reverse phase chromatography to give (S)-1-(N-isopentyl-N-methylcarbamoyl)-2-(4-tert-butoxyhydroxyphenyl)ethylamino 2-(4-(tert-butoxycarbonyl)piperazin-1-yl)ethyl formate (262 mg, 54%) a...
Embodiment 2
[0239] [(1S)-2-[Benzyl(methyl)amino]-1-(4-hydroxybenzyl)-2-oxoethyl]carbamate 2-piperazin-1-ylethyl ester dihydrochloride
[0240]
[0241] (S)-1-(carboxy)-2-(4-tert-butoxyphenyl)ethylcarbamate 2-(4-(tert-butoxycarbonyl)piperazin-1-yl)ethyl ester (intermediate Body 1; 378 mg, 0.77 mmol), N-methylbenzylamine (95 mg, 0.75 mmol), PyBrOP (360 mg, 0.77 mmol) and DIPEA (0.40 mL, 2.30 mmol) were dissolved in DMF (10 mL) cooled with ice water . The reaction mixture was stirred overnight, then concentrated in vacuo. Suspend the residue in 6% NaHCO 3 in aqueous solution (50 mL) and extracted with DCM (3 x 50 mL). The combined DCM extracts were dried (MgSO 4) and concentrated in vacuo. Purification of the residue by normal phase chromatography (gradient elution with MeOH in DCM from 0% to 10%) followed by reverse phase chromatography afforded (S)-1-(N-benzyl-N-methylaminomethyl 2-(4-(tert-butoxycarbonyl)piperazin-1-yl)ethyl)-2-(4-tert-butoxyphenyl)ethylcarbamate (164 mg, 35%), y...
Embodiment 3
[0244] {(1S)-1-(4-Hydroxybenzyl)-2-[methyl(2-phenylethyl)amino]-2-oxoethyl}carbamic acid 2-piperazin-1-ylethyl ester di Hydrochloride
[0245]
[0246] (S)-1-(carboxy)-2-(4-tert-butoxyphenyl)ethylcarbamate 2-(4-(tert-butoxycarbonyl)piperazin-1-yl)ethyl ester (intermediate Body 1; 404mg, 0.82mmol), N-methylphenethylamine (120mg, 0.89mmol), PyBrOP (390mg, 0.84mmol) and DIPEA (0.4mL, 2.3mmol) were dissolved in DMF (10mL )middle. The reaction mixture was stirred overnight then concentrated in vacuo. Suspend the residue in 6% NaHCO 3 in aqueous solution (50 mL) and extracted with DCM (3 x 50 mL). The combined DCM extracts were dried (MgSO 4 ) and concentrated in vacuo. Purification of the residue by reverse phase chromatography gave (S)-1-(N-methyl-N-phenethylcarbamoyl)-2-(4-tert-butoxyphenyl)ethylcarbamate 2-( 4-(tert-butoxycarbonyl)piperazin-1-yl)ethyl ester (257 mg, 51%) as a yellow gum. All of this material (257 mg, 0.42 mmol) was dissolved in DCM (10 mL), treated wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap