21(r) Use of Argatroban
A technology of pharmaceutical excipients and drugs, applied in the field of medicine, can solve the problems of low biological activity, poor therapeutic effect and high dosage
Inactive Publication Date: 2011-11-30
TIANJIN WEIJIE TECH
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] However, at present, a mixture of 21(R) argatroban and 21(S) argatroban is commonly used clinically. Generally speaking, the drug mixture has lower biological activity than a single-component drug, and the therapeutic effect is poor and the dosage is high. Moreover, in recent years, countries around the world have increasingly s
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
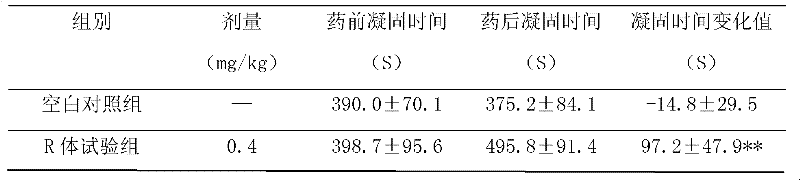
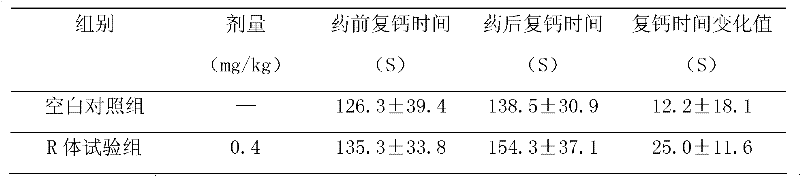
An application of 21(R) argatroban in the treatment and prevention of thrombosis and platelet aggregation inhibitory drugs. The medicine includes 21(R) argatroban as a single component and pharmaceutical auxiliary materials. The 21 (R) argatroban of the single component provided by the present invention has significant effect on prolonging normal canine whole blood coagulation time (CT), and its effect is 7 times that of the blank control group; The recalcification time (RT) of normal dogs has a significant effect, and its effect is twice that of the blank control group; 21 (R) argatroban has a significant effect on prolonging the prothrombin time (PT) of normal dogs, and its effect is more than that of the blank control group 15 times of that of the control group; 21 (R) argatroban has a certain effect on prolonging the normal canine kaolin partial thromboplastin time (APTT) and prolonging the normal canine thrombin time (TT), and its effect is slightly stronger than that of the blank control group ; 21 (R) Argatroban can reduce platelet adhesion rate and platelet aggregation rate in normal dogs.
Description
technical field [0001] The invention belongs to the technical field of medicine, in particular to the application of a direct thrombin inhibitor 21(R) argatroban. Background technique [0002] In 1978, the antithrombin activity of Argatroban (Argatroban) monohydrate [US 4 101 653] was first reported by S.Okamoto of Japan Mitsubishi Chemical Company. During the following 20 years, many researchers conducted in-depth research on the biological activity of argatroban and its value as medicine. In 1981, S.Okamoto compared the drug with heparin in animals [Okamoto, S.et al., Biochem.Biophys.Res.Commun.101, 440 (1981)]; T.Kumoto et al. Activity was reported [Kumada, T.et al., Thromb.Res.24, 285 (1981)]; in 1984, R.Kumato carried out clinical hemodialysis evaluation [Kikumoto, R.et al., Biochemistry 23,85 (1984)]; In 1986, the author reported that argatroban inhibited thrombin activity in mammals and could be used as a therapeutic and prophylactic agent for thrombosis and an inhi...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K38/05A61P7/02
Inventor 宋洪海张冉高巨孙致远
Owner TIANJIN WEIJIE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com